
Research Article
Austin Alzheimers J Parkinsons Dis. 2014;1(1): 7.
Long Duration Exercise Program in Individuals with Parkinson’s Disease: Effects on Functional Capacity
Baptista AM1, Gobbi LTB1, Beretta VS1, Vitório R1, Teixeira-Arroyo C1, Lirani-Silva E1, Stella F1,3 and Barbieri FA2*
1Department of Physical Education, University of Estadual Paulista,UNESP Rio Claro, Brazil
2Department of Physical Education, University of Estadual Paulista, UNESP Bauru, Brazil
3UNICAMP – Campinas State University at Campinas, Brazil
*Corresponding author: Barbieri FA, Department of Physical Education, Information, Vision and Action Laboratory, University of Estadual Paulista, UNESP, Faculty of Science - Bauru,SP, Brazil.
Received: August 06, 2014; Accepted: August 29, 2014; Published: August 30, 2014
Abstract
The aim of this study was to investigate the effects of long duration exercise program on physical fitness components of functional capacity in individuals with Parkinson disease (PD) and to evaluate ongoing effects of exercise after 8 to 10-week follow-up without exercise. Twenty-four individuals with PD were randomly assigned to two groups: generalized exercise program and stretching exercise program (control group). The generalized exercise program provided training in physical fitness components of functional capacity. The stretching exercise program was characterized by low intensity and volume, mainly with static exercises. Both groups were evaluated before (BI) and after the 4-month (AI) exercise program. In addition, the individuals of generalized exercise program were also evaluated after 8-month exercise program and after 8 to 10- week follow-up without exercise. The generalized exercise program improved flexibility (BI - 38.50±12.42 cm; AI - 44.00±12.74 cm) and agility (BI - 30.59±7.54 s; AI - 28.56±8.20 s) while the stretching exercise program worsened coordination (BI - 23.27±6.58 s; AI - 28.06±7.37 s) and aerobic resistance (BI- 13.64±3.76 min; AI - 17.27±5.15 min) and improved balance (BI - 44.00±7.79 pts; AI - 46.57±6.53 pts). Lower-limb strength and UPDRS-motor scale scores were better at 8 months (14.75±2.92 rep and 26.25±13.97 pts, respectively) compared to baseline (13.13±2.59 rep and 31.63±12.82 pts, respectively) and 4 months (13.50±1.93 rep and 30.38±14.52 pts, respectively) for generalized exercise program. However, the benefits of 8 months of exercise were lost after 8 to 10-week follow-up without exercise (lower-limb strength - 12.43±3.15 rep and UPDRS-motor scale - 32.57±14.05 pts). In conclusion, generalized exercise program improved the functional capacity in individuals with PD, differently of stretching exercise program. In addition, a long duration exercise program promoted benefits for functional capacity and disease progression in individuals with PD. However, benefits were lost after a short period without exercise.
Keywords: Exercise program; Long duration; Functional capacity; Parkinson disease
Introduction
Exercise and pharmacological treatment are considered the most efficient strategies to manage the symptoms of Parkinson’s disease (PD) [1-3]. Despite importance of pharmacological treatment to reduce the motor and non-motor symptoms [4], many collateral effects are caused by the prolonged use of PD medicines [5,6]. On the other hand, exercise programs have no collateral effects and are able to improve multiple dimensions of the quality of life in individuals with PD [7], such as balance and mobility [8-11], motor symptoms [10,12,13] and mainly functional capacity [14-16], which is the primary symptom to impair in PD [17]. The progressive reduction of functional capacity occurs due to impaired basal ganglia having an inadequate effect on the cortical motor centers [18-20]. Longterm exercise benefits brain functioning by increasing the blood and oxygen flow to the brain [21], promoting neurogenesis and synaptic plasticity [2], and facilitating motor performance through release of neurotransmitters, such as dopamine [1,2]. However, little is known about the effects of long duration exercise on functional capacity in individuals with PD, mainly related to the optimal content of exercise programs (intensity, dosing and component exercises) [3,14].
Effects of short duration exercise programs (from 8 to 12 weeks) on functional capacity are well known [22-24]. The main adaptations and improvements of exercise programs happened in the first months of exercise program [25]. However, exercise practice should not be promoted only as a therapy during some weeks or months, but as an activity of a healthy individual lifestyle [8]. Although it is well documented that physical activity should be a component of healthy everyday life for everyone [26,27], there are in the literature few studies that prescript exercise programs by more than 4 months uninterrupted (long duration exercise programs) and totally supervised for individuals with PD [8-11,14,16]. Only Orcioli-Silva and collaborators [14] and States and collaborators [16] investigated specifically the effects of long duration exercise program on physical fitness components of functional capacity, such as aerobic endurance, coordination, flexibility and strength. Moreover, previous studies did not consider the benefits of the exercise after a period without exercise (follow-up). Therefore, new studies are necessary to help in the prescription of long duration exercise programs for individuals with PD, especially for functional capacity.
The aim of this study was to investigate the effects of long duration exercise program on functional capacity in individuals with PD and to evaluate ongoing effects of exercise after 8 to 10-week follow-up without exercise. We compared two types of 4-month exercise programs: generalized exercise program and stretching exercise program (control group). In addition, we analyzed the effects of 8-month generalized exercise program and the ongoing benefits after 8 to 10-week follow-up. The hypotheses of the study were: i) the individuals who participated on generalized exercise program would improve more the components of functional capacity, such as balance, resistance and strength, than individuals who participated on stretching exercise program; ii) 8 months of exercise would promote more improvements on the components of functional capacity than 4 months; iii) the benefits of 8 months of exercise after 8 to 10-week follow-up.
Materials and Methods
Twenty-four individuals with PD participated in this study (Table 1), who participated in a multidisciplinary project in PD conducted at São Paulo State University at Rio Claro (UNESP-RC). They were clinically examined by an expert physiatrist (FS), who confirmed the diagnosis of PD. The inclusion criteria included individuals had a clinically confirmed diagnosis of PD scored between I and III on the Hoehn& Yahr rating scale [28], regular intake of PD drugs, and being able to participate in the exercise independently. All of the participants provided written informed consent to participate in this study, which was approved by the local institutional review board. The participants were randomly assigned to, according Hoehn & Yahr scale and age, two groups: generalized exercise program and stretching exercise program (control group). Each group was composed by 12 individuals with idiopathic PD.
Generalized exercise program
Stretching exercise program
p-values
Gender
7 males (n=12)
6 males (n=12)
Age (years)
68.50±6.93 (79 - 55)
74.00±6.24 (83 - 60)
0.09
Age at onset (years)
62.50±9.85 (77 - 46)
68.00±10.17 (80 - 45)
0.15
Body weight (kg)
62.55±7.56 (75.8 - 45.2)
71.60±15.96 (90 - 51)
0.43
Body height (m)
1.59±0.05 (1.71 - 1.47)
1.50±0.06 (1.7 - 1.4)
0.10
Table 1: Medians, standard deviations and ranges of demographic characteristics of generalized exercise program and stretching exercise program.
All individuals were evaluated before commencing the exercise program (Figure 1). For both exercise programs, the individuals who participated in 75% of exercise program sessions were evaluated after 4 months of exercise. Eight individuals and 7 individuals participated in 75% or more sessions of the generalized and stretching exercise program, respectively. The individuals of generalized exercise program participated in more 4 months of this exercise program (totalizing 8 months of exercise) and were evaluated after this period. All individuals participated in 75% or more sessions in this part of the exercise program. In addition, these individuals were evaluated again after 8 to 10 weeks of follow-up without exercise. One individual did not participate in this evaluation.
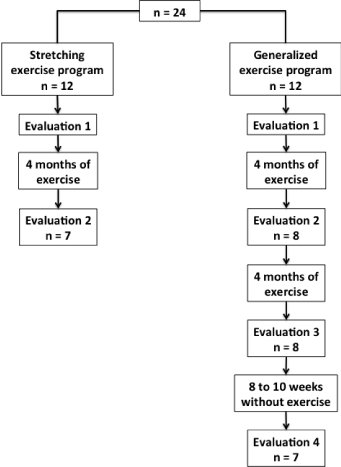
Figure 1: Consort flow diagram illustrating study design.
The participants underwent health screening, clinical evaluation and functional capacity evaluation between 8.00 and 11.00 a.m. due to participants medicines schedules. All participants were evaluated during the “on” state of medication. During the clinical evaluation,data were collected using demographic and anthropometrical guidelines that included: gender, age, age at disease onset, medicines, and body weight and height (Table 1). In addition, the psychiatric physician assessed Parkinson motor symptoms and signs, mental status and PD severity using UPDRS - motor examination [29], Mini- Mental State Examination [30] and modified Hoehn and Yahr Scaling for PD, respectively. For the functional capacity evaluation [31], the physical fitness components were assessed over 2 days, using the test proposed by the American Alliance for Health Physical Education Recreation and Dance (AAHPERD) [32], the Berg Balance Scale [33] and sit-to-stand test [34]. The AAHPERD test includes measures of flexibility, muscular strength, agility, coordination, and aerobic endurance. The Berg Balance Scale and sit-to-stand test were used to assess global functional balance ability and lower-limb strength, respectively.
The generalized exercise program provided training in flexibility, coordination, strength (upper and lower limbs), aerobic resistance and balance to improve the functional capacity. The exercises were associated with cognitive and sensorial activities. Gymnastics and strength training were planned to improve upper and lower limbs strength, especially of the large muscle groups; rhythmic activities were designed to train motor coordination; passive and active stretching with and without materials was used to train flexibility; circuits, walking, trekking, ladder and ramps were used to train aerobic resistance (the hearth rate was controlled by heart rate monitor); and leisure activities were included to train static and dynamic balance. The generalized exercise program took place over 8-month period with 96 sessions, 3 times a week and 60 minutes per session. Each session consisted of five parts: warm-up (5 minutes), pre-exercise stretching (5 minutes), exercise session (40 minutes), cool down (5 minutes) and post-exercise stretching (5 minutes).
The exercise program was structured into six phases (Figure 2a). Each phase consisted of 4 cycles with 4 sessions. At the end of each phase there was a progressive load (intensity or volume of load and in complexity of the exercise increment) according each physical fitness components of functional capacity (Figure 2b).
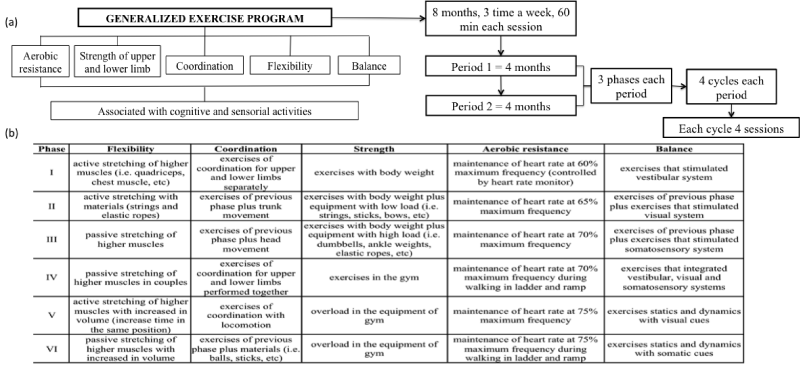
Figure 2: (a) Description of generalized exercise program and (b) The overload for each component of functional capacity.
The stretching exercise program aimed to train flexibility. The main characteristics of this program were low intensity and volume, mainly with static positions (standing and sitting positions). The stretching exercise program took place over 4–month period with 48 sessions, 3 times a week and 60 minutes per session. Each session consisted of three parts: warm-up (5 minutes), stretching session (40 minutes) and cognitive session (15 minutes). The exercise program was structured into three phases of 4 cycles with 4 sessions. At the end of each phase there was a progressive load (complexity and position for stretching, and complexity for cognitive aspect). The characteristic of three phases were:
- Phase I – stretching of big muscles of upper and lower limb, trunk and neck. The exercises were performed in sitting position. In the first session was stretched the upper-limb and trunk, in the second session was stretched the lower-limb and neck, and in the third session was stretched all parts of the body. The individuals performed 3 series of each exercise with 10 seconds of duration and 10 seconds of rest. For cognitive aspect were used activities involving memory.
- Phase II – the focus was upper-limbs with increased of complexity in comparison to phase I. The exercises were performed in standing position. In the first session was stretched the upper-limb and trunk, in the second session was stretched all parts of the body with focus in the lower-limb, and in the third session was stretched the upper-limb and trunk again. In this phase, the individuals performed 4 series of each exercise with 15 seconds of duration and 10 seconds of rest. For cognitive aspect was included activities involving attentionand increased the complexity of memory activities.
- Phase III - the focus was lower-limbs with increased of complexity in comparison to phase I. The exercises were performed in standing position and in one-leg balance. In the first session was stretched the lower-limb and neck, in the second session was stretched all parts of the body with focus in the upper-limb, and in the third session was stretched the lower-limb and neck again. The same intensity and volume of previous phase was used in this phase. For cognitive aspect was increased the complexity of attention activities.
Both exercise programs kept the same schedule (8:30 am to 9:30 am). Patients took PD drugs one hour before the start of the session. Therefore, medicines were controlled for each patient every month to avoid effects on exercise program. At least three physical education professionals supervised each exercise program in each session.
SPSS for Windows 15.0® was used to statistically process the data (p<0.05). The Shapiro–Wilk test was used to test the normality assumption. To test the first hypothesis of the study (higher improvements for the individuals that participated on generalized exercise than individuals that participated on stretching exercise program), the clinical variables and functional capacity parameters were analyzed by ANOVA two-way with factor for group (generalized exercise program x stretching exercise program) and time (evaluation 1 x evaluation 2), with repeated measures of the last factor. To test the second hypothesis (8 months of exercise would promote more improvements in the components of functional capacity than 4 months), the clinical variables and functional parameters were analyzed by ANOVA one-way with factor for time (evaluation 1 x evaluation 2 x evaluation 3), with repeated measures. To test the third hypothesis (individuals would maintain the benefits of 8-month exercise program after 8 to 10-week follow -up), the clinical variables and functional parameters were analyzed by ANOVA one-way with factor for time (evaluation 3 x evaluation 4), with repeated measures. For the two first ANOVAs, when the analyses pointed out significant interactions, Tukey univariate tests were carried out.
Results
The groups before intervention showed no differences for demographic characteristics and clinical variables (Table 1 and 2). Both groups showed no effects of intervention for clinical variables after 4 months of exercise (Table 2). For physical fitness components of functional capacity, ANOVA indicated main effects of group and evaluation. Moreover, there were group x time interaction for flexibility, agility, aerobic resistance and balance. For group factor, the individuals that participated on generalized exercise program showed better agility, aerobic resistance, lower-limb strength and balance. For time, 4-month exercise program improved flexibility, agility and balance, but coordination was worsened. Regarding the group x time interactions, the generalized exercise program improved flexibility (p<0.003) and agility (p<0.003) of the individuals with PD after 4 months of exercise. Coordination (p<0.02) was worsened while other physical fitness components showed no significant differences after 4 months of exercise. As concerning the stretching exercise program, coordination (p<0.001) and aerobic resistance (p<0.007) was worsened and balance (p<0.01) was improved after 4 months of exercise.
Generalized Exercise Program
Stretching Exercise Program
Group p-values
Time p-values
Interaction p-values
Before intervention
After intervention
Before intervention
After intervention
Clinical variables
HY scale
1.81±0.86
2.00±0.80
2.07±0.93
2.29±0.91
0.55
0.07
0.89
UPDRS-motor (pts)
31.63±12.82
30.38±14.52
29.29±14.50
29.43±13.18
0.81
0.73
0.66
MEEM (pts)
26.75±3.20
26.50±3.59
24.43±2.88
25.14±3.44
0.28
0.64
0.34
Functional parameters
Flexibility (cm)
38.50±12.42
44.00±12.74
45.30±8.98
46.07±9.99
0.45
0.01
0.05
Coordination (s)
17.43±5.75
20.13±6.10
23.27±6.58
28.06±7.37
0.06
0.001
0.04
Agility (s)
30.59±7.54
28.56±8.20
54.03±19.54
52.82±20.48
0.01
0.02
0.03
Upper-limb strength (rep)
17.88±3.52
17.13±2.36
14.43±4.28
17.00±3.32
0.24
0.37
0.11
Aerobic resistance (min)
9.51±1.36
9.07±1.10
13.64±3.76
17.27±5.15
0.001
0.06
0.02
Lower-limb strength (rep)
13.13±2.59
13.50±1.93
8.86±4.67
9.14±4.10
0.02
0.65
0.95
Balance (pts)
52.38±3.70
53.38±2.20
44.00±7.79
46.57±6.53
0.01
0.01
0.03
Table 2: Means and standard deviations of clinical variables and functional parameters for generalized exercise program and stretching exercise program before and after 4 months of intervention. The last three columns show the p-values for main effects of group, time and group x time interaction, respectively.
Long duration (8 months) of generalized exercise program improved lower-limb strength (p<0.04) compared with baseline (Table 3). None of physical fitness components of functional capacity worsened after 8-month exercise program. In addition, the generalized exercise program was able to improve the UPDRS-motor (p<0.04) after 8 months of exercise. However, the benefits were not maintained after 8 to 10-week without exercise. The other physical fitness components and clinical variables showed no differences after 8 to 10-week follow-up.
Before intervention
After 4 months
After 8 months
Follow-up (8-10 weeks)
Time p-Values
Follow-up p-values
Clinical variables
HY scale
1.81±0.88
2.00±0.80
1.94±0.82
2.00±0.87
0.26
0.76
UPDRS-motor (pts)
31.63±12.82
30.38±14.52
26.25±13.97
32.57±14.05
0.05
0.04
MEEM (pts)
26.75±3.20
26.50±3.59
26.00±3.66
26.82±3.58
0.47
0.63
Functional parameters
Flexibility (cm)
38.50±12.42
44.00±12.74
42.13±13.65
42.57±11.75
0.03
0.19
Coordination (s)
17.43±5.75
20.13±6.10
19.92±7.56
19.59±6.62
0.04
0.69
Agility (s)
30.59±7.54
28.56±8.20
30.09±13.99
30.63±8.26
0.05
0.98
Upper-limb strength (rep)
17.88±3.52
17.13±2.36
17.88±3.52
17.14±2.85
0.69
0.47
Aerobic resistance (min)
9.51±1.36
9.07±1.10
9.66±1.80
9.95±1.47
0.31
0.90
Lower-limb strength (rep)
13.13±2.59
13.50±1.93
14.75±2.92
12.43±3.15
0.02
0.03
Balance (pts)
52.38±3.70
53.38±2.20
53.63±2.92
52.29±3.09
0.09
0.13
Table 3: Means and standard deviations of clinical variables and functional parameters for generalized exercise program before, after 4 months and after 8 months of intervention and follow-up after 8 to 10-week. The last two columns show the p-values for main effects of time and follow-up, respectively.
Discussion
We investigated the effects of long duration exercise program on functional capacity in individuals with PD. The generalized exercise program improved (or maintained) the level of physical fitness components of functional capacity, mainly flexibility and agility, while the stretching exercise program (control group) worsened coordination and aerobic resistance and improved balance. Therefore, our first hypothesis (higher improvements for the individuals that participated on generalized exercise than individuals that participated on stretching exercise program) was confirmed in part. The improvement of balance at control group seems to be explained by the effects of passive stretching of antagonist muscles and agonist muscles [35] and the maintenance of balance positions, similar to Tai Chi [36]. Interestingly, 8 months of exercise improved only lower-limb strength compared with baseline and 4 months of exercise. However, none physical fitness components of functional capacity worsened after 8 months of exercise compared with baseline and 4 months of exercise. The most relevant finding in long duration exercise program was the improvement of UPDRS-motor scale, which seems to indicate a general motor improvement, corroborating to second hypothesis (8 months of exercise would promote more improvements in the components of functional capacity than 4 months). However, the benefits of 8 months of exercise were not maintained after 8 to 10-week follow-up, which is contrary to third hypothesis (individuals would maintain the benefits of 8 months of exercise after 8 to 10-week follow-up).
Generalized exercise program is able to combat the effects of PD on functional capacity, which corroborated with previous studies [14-15,37]. Individuals with PD have shown reduction on functional capacity more quickly than their healthy peers [38]. The impairments of PD in basal ganglia have an inadequate effect on the cortical motor centers, which in turn lead to less activation of motor neurons [18,19]. However, exercise seems to step (improve) in the integration and processing of somatosensory information, encouraging greater structural adaptation [39]. Fox and collaborators [39] suggest that exercise enhance functional capacity due to intensive and complex activity maximizes synaptic plasticity and promotes greater structural adaptation, respectively. In addition, exercise that are rewarding increases dopamine levels, which is highly responsive to exercise and inactivity (“use it or lose it”) [39]. Therefore, exercise improves quality of life and ability to perform daily activities of individuals with PD.
We found two important findings: 1) Agility improved after generalized exercise program. Improvement in agility is directly related to bradykinesia symptom. Berardelli, and collaborators [40] have suggested that improvement in bradykinesia occurs due to greater integration of sensory information by the basal ganglia, which results in better motor planning and greater muscle recruitment to initiate movement that seems to happen with agility improvement. Therefore, the individuals with PD decrease risk of falls and dependence, improving locomotion [8,41]; 2) Aerobic resistance was maintained after generalized exercise program (4 months and 8 months of exercise), but not after stretching exercise program (4 months of exercise). The low intensity and volume characteristic of the stretching exercise program promoted a low dose of physical activity, which may not be considered a form of exercise, such as physiotherapy [3]. Previous study also found maintained (modest benefit) for aerobic resistance after long-term exercise [16]. A good aerobic resistance improves functional capacity, reduces functional decline and contributes to independent and health lifestyle [26,27]. However, individuals with PD already have a lower maximum capacity compared to neurologic healthy people [42]. Low cardiorespiratory capacity may trigger degenerative diseases, such as hypertension, diabetes, heart problems, and others [26]. Despite beneficial findings, the coordination worsened following both generalized and stretching exercise programs, which are contrary previous studies [14]. However, Orcioli-Silva and collaborators [14] found improvements of coordination after exercise in individuals with PD in the bilateral disease. Our findings could be explained by the individuals of generalized exercise program was unilateral stage of the disease, which did not show improvement in previous study [14] and the control group did not perform a specific training for coordination, which was expected a maintained or worsened after 4 months. In addition, slowness of movement arising from bradykinesia and akinesia could explain lower performance in the coordination [32].
Long duration exercise program improves motor function in PD. Previous studies with short duration exercise program show little effects of exercise on clinical aspects [12,13,43]. Our finding confirms previous studies that indicated improvement of UPDRSmotor after long duration exercise program [10,11], which indicates improvements from 10% to 30% (we found 16%). The improvements in UPDRS-motor suggest that generalized long duration exercise programs seem to have a disease modifying effect. Shulman and collaborators [44] suggested that strong exercise programs are more indicated to improve the UPDRS score. Therefore, long duration exercise program promotes behavior change, which is important for disease progression and seems no to happen in short duration exercise programs. However, this finding may be analyzed carefully because this could just be reflective of the timing of assessments.
A brake of exercise seems no ongoing to maintain the benefits on functional capacity and disease progression in individuals with PD. After 8 to 10-week follow-up all clinical variables and functional parameters showed low values (not significant) in comparison to 8-month exercise program. Lower-limb strength and UPDRS-motor were the aspects that showed significant worsening after follow-up. Basic and clinical researches suggest that the intensity property of exercise (i.e. repetitiveness, velocity and complexity) may contribute to activity-dependent neuroplasticity, central nervous system alterations in response to physical activity, such as neurogenesis of damaged brains [45]. These findings confirm that individuals with PD require participation in exercise programs without interruption.
Even with consistent and relevant results, this study has some limitations. The individuals were not double blind because it is not possible to blind participants with respect to exercising and to the specific exercises they are performing. In addition, the sample was small, which can affect our power to detect differences in some measures. Finally, the control group participated only 4 months of exercise in the study. Our findings showed worsening on functional capacity after 4-month stretching exercise program. Probably the functional capacity would be more deteriorated after 8 months with low intensity exercise. Thus, we decided to include the individuals in an exercise program similar to generalized exercise program. In addition, despite the low intensity and volume of the stretching exercise program, we did not have a group (control) that did not exercise, which is recommend in future studies. Therefore, the findings should be interpreted with care.
Conclusion
In conclusion, generalized exercise program improved the physical fitness components of functional capacity in individuals with PD, differently of stretching exercise program. In addition, a long duration exercise program promoted benefits for functional capacity and disease progression in individuals with PD, especially for UPDRS-motor. However, a short period (8 to 10 weeks) without exercise seems no ongoing maintenance the benefits. Finally, exercise should be introduced at an early stage of the disease to slow its progression [14] and maintained in the diary routine, improving corticospinal motor excitability and neuroplasticity [39], and helping to attenuate the effects of medication [1,2].
References
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord. 2010; 25: S141-145.
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007; 27: 5291-5300.
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008; 23: 631-640.
- Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson's disease. CNS Drugs. 2007; 21: 677-692.
- Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000; 23: S2-7.
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009; 72: S1-136.
- Zesiewicz TA, Evatt ML. Potential influences of complementary therapy on motor and non-motor complications in Parkinson's disease. CNS Drugs. 2009; 23: 817-835.
- Gobbi LT, Oliveira-Ferreira MD, Caetano MJ, Lirani-Silva E, Barbieri FA, Stella F, et al. Exercise programs improve mobility and balance in people with Parkinson's disease. Parkinsonism Relat Disord. 2009; 15: S49-S52.
- Pereira MP, Ferreira MDTO, Caetano MJD, Vitório R, Lirani-Silva E, Barbieri FA, et al. Long-Term multimodal exercise program enhances mobility of patients with Parkinson’s disease. ISRN Rehabilitation. 2012: 1-7.
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JC, et al. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Mov Disord. 2010; 25: 1217-1225.
- Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012; 26: 132-143.
- Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson's disease. Mov Disord. 2009; 24: 1132-1138.
- Sage MD, Almeida QJ. A positive influence of vision on motor symptoms during sensory attention focused exercise for Parkinson's disease. Mov Disord. 2010; 25: 64-69.
- Orciolo-Silva D, Barbieri FA, Simieli L, Rinaldi NM, Vitório R, Gobbi LTB. Effects of a multimodal exercise program on the functional capacity of Parkinson's disease patients considering disease severity and gender. Motriz. 2014; 20: 100-106.
- Cyarto EV, Brown WJ, Marshall AL, Trost SG. Comparison of the effects of a home-based and group-based resistance training program on functional ability in older adults. Am J Health Promot. 2008; 23: 13-17.
- States RA, Spierer DK, Salem Y. Long-term group exercise for people with Parkinson's disease: a feasibility study. J Neurol Phys Ther. 2011; 35: 122-128.
- Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011; 123: 73-84.https://journals.lww.com/jnpt/Citation/1997/21040/A_Rationale_for_Strength_Training_in_Patients_with.10.aspx
- Glendinning DS, Enoka RM. Motor unit behavior in Parkinson's disease. Phys Ther. 1994; 74: 61-70.
- Canning CG, Alison JA, Allen NE, Groeller H. Parkinson's disease: an investigation of exercise capacity, respiratory function, and gait. Arch Phys Med Rehabil. 1997; 78: 199-207.
- Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur J Phys Rehabil Med. 2009; 45: 215-229.
- Yang YR, Lee YY, Cheng SJ, Wang RY. Downhill walking training in individuals with Parkinson's disease: a randomized controlled trial. Am J Phys Med Rehabil. 2010; 89: 706-714.
- Scandalis TA, Bosak A, Berliner JC, Helman LL, Wells MR. Resistance training and gait function in patients with Parkinson's disease. Am J Phys Med Rehabil. 2001; 80: 38-43.
- Lee KS, Lee WH, Hwang S. Modified constraint-induced movement therapy improves fine and gross motor performance of the upper limb in Parkinson disease. Am J Phys Med Rehabil. 2011; 90: 380-386.
- Wilmore JH, Kenney WL. Physiology of Sport and Exercise. 4th edn. Champaign: Human Kinetics. 2007.
- Erikssen G. Physical fitness and changes in mortality: the survival of the fittest. Sports Med. 2001; 31: 571-576.
- American College of Sports Medicine. Guidelines for exercise testing and prescription. Baltimore: Lippincott Williams. 2000.
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004; 19: 1020-1028.
- Fahn S, Elton R. Members of the UPDRS Development Comitee the Unified Parkinson's Disease Rating Scale. Fahn S, Marsden CD, Calne DB, Goldstein N, editors. Recent developments in Parkinson's disease. Mcmellam Health Care Information. 1987; 153-163.
- Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. [Suggestions for utilization of the mini-mental state examination in Brazil]. Arq Neuropsiquiatr. 2003; 61: 777-781.
- Barbieri FA, Rinaldi NM, Santos PC, Lirani-Silva E, Vitório R, Teixeira-Arroyo C, et al. Functional capacity of Brazilian patients with Parkinson's disease (PD): relationship between clinical characteristics and disease severity. Arch Gerontol Geriatr. 2012; 54: e83-88.
- Osness WH, Adrian M, Clark B, Hoeger W, Raab D, Wiswell R. Functional fitness assessment for adults over 60 years: a field based assessment. Reston: The American Alliance for Health, Physical Education, Recreation and Dance. 1990.
- Scalzo PL, Nova IC, Perracini MR, Sacramento DR, Cardoso F, Ferraz HB, et al. Validation of the Brazilian version of the Berg balance scale for patients with Parkinson's disease. Arq Neuropsiquiatr. 2009; 67: 831-835.
- Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997; 78: 26-32.
- Gracies JM. [Neurorehabilitation in parkinsonian syndromes]. Rev Neurol (Paris). 2010; 166: 196-212.
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012; 366: 511-519.
- Dibble LE, Hale TF, Marcus RK, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: A preliminary study. Parkinsonism Relat Disord. 2009; 15: 752-757.
- Fertl E, Doppelbauer A, Auff E. Physical activity and sports in patients suffering from Parkinson's disease in comparison with healthy seniors. J Neural Transm Park Dis Dement Sect. 1993; 5: 157-161.
- Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin Speech Lang. 2006; 27: 283-299.
- 40. Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001; 124: 2131-2146.
- Vitório R, Teixeira-Arroyo C, Lirani-Silva E, Barbieri FA, Caetano MJ, Gobbi S, et al. Effects of 6-month, Multimodal Exercise Program on Clinical and Gait Parameters of Patients with Idiopathic Parkinson's Disease: A Pilot Study. ISRN Neurol. 2011; 2011: 714947.
- Carter JH, Nutt JG, Woodward WR. The effect of exercise on levodopa absorption. Neurology. 1992; 42: 2042-2045.
- Hackney ME, Earhart GM. Short duration, intensive tango dancing for Parkinson disease: an uncontrolled pilot study. Complement Ther Med. 2009; 17: 203-207.
- Shulman LM, Bhat V. Gender disparities in Parkinson's disease. Expert Rev Neurother. 2006; 6: 407-416.
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol (1985). 2006; 101: 1776-1782.