
Research Article
Austin Alzheimers J Parkinsons Dis. 2014;1(3): 4.
Initial Experience with Levodopa Intestinal Gel for Advanced Parkinson’s Disease
Cohen OS1,3*, Yahalom G1, Strauss H1, Orlev Y1, Faust-Socher A1, Benizri S1, Lang A,2, Lerman SF1,4 and Hassin-Baer S1,3
1Department of Neurology, Parkinson’s Disease and Movement Disorders Clinic, Chaim Sheba Medical Center, Israel
2Department of Gastroenterology, Chaim Sheba Medical Center, Israel
3Sackler Faculty of Medicine, Tel-Aviv University, Israel
4Ben-Gurion University, Israel
*Corresponding author: Cohen OS, Department of Neurology, Parkinson’s Disease and Movement Disorders Clinic, Chaim Sheba Medical Center and the Sagol Neuroscience Center, Hashomer 52621, Israel.
Received: October 29, 2014; Accepted: November 10, 2014; Published: November 11, 2014
Abstract
Background: Continuous infusion of Levodopa/Carbidopa Intestinal Gel (LCIG) by a portable pump, directly to the duodenum, via Percutaneous Endoscopic Gastrojejunostomy (PEG) is a new treatment option for patients with advanced Parkinson’s disease (PD) that has recently become available in Israel.
Objectives: To report our initial experience concerning, efficacy, safety and feasibility of LCIG treatment
Methods: Consecutive patients with advanced PD experiencing motor fluctuations despite optimized pharmacologic therapy or deep brain stimulation were included. Clinical assessments included the Unified PD Rating Scale (UPDRS), the Clinical Global Impression of Change (CGI-C), and patient and caregiver interview.
Results: Between January 2012 and August 2013, 17 PD patients went through the first treatment phase (LCIG titration via a naso-duodenal tube); ten of them (6 males, age 67.3±9.5 years, range 55-87, PD duration 16.1±5.8 years) proceeded to PEG placement and permanent treatment phase. The mean follow-up time was 11.1±8.5 months (range 3-22 months). Five patients had an improvement of at least 25% in the motor “on” UPDRS score. All patients reported significant reduction of daily “off” time, and improvement in dyskinesia duration and disability. Physicians, patients and caregivers reported moderate-marked global improvement as rated by the CGI-C. The procedure and treatment were generally well tolerated despite transient procedure and device-related adverse events occurring in 6 patients.
Conclusion: The initial experience with LCIG in our center has been positive as both the procedure and treatment proved to be safe and very beneficial for the motor features and global well-being of advanced PD patients.
Keywords: Parkinson’s disease; Continues dopaminergic stimulation (CDS); Levodopa/carbidopa intestinal gel(LCIG)
Introduction
Parkinson’s disease (PD) is the second most common human neurodegenerative disorder whose prevalence is 1%-2% in people aged 60 years or older [1]. Since its discovery in the 1960’s, levodopa (along with carbidopa or benserazide; a peripheral amino acid decarboxylase inhibitor) is the gold standard of symptomatic treatment for PD due to its dramatic beneficial effect [2]. However within a few years, after an initial “honeymoon period”, the beneficial effect is often compromised by the occurrence of shortening of response to levodopa with disabling fluctuations and dyskinesia. The main manifestations of the fluctuations include profound changes in the motor state with prolonged episodes of tremor, akinesia and rigidity as well as anxiety, mental dullness, depression and pain during the day [3]. The reasons for their occurrence are diverse and include both central and peripheral factors; at the early stages of PD, the still-intact remaining dopamine nerve terminals buffer the periodic administration and rapid metabolism of orally administered levodopa and enable Continuous Dopaminergic Stimulation (CDS) and a smooth clinical response [4]. However in advanced PD the regulatory function by the residual dopaminergic neurons is lost [5] and non-physiologic pulsatile stimulation occurs. Clinical fluctuations are also closely related to fluctuations in levodopa plasma levels resulting from peripheral pharmacokinetic factors including impaired gastric emptying and competition with dietary protein for levodopa absorption [6].
Optional management strategies for the motor fluctuations include changes in medication regimen and diet, such as frequent administration of low doses of levodopa, on an “empty stomach”, minimizing protein content in meals,. Controlled release levodopa preparations or addition of other antiparkinsonian medications, such as dopamine agonists, Monoamine Oxidase Type B (MAO-B) inhibitors, and Catechol-O-Methyl Transferase (COMT) inhibitors. When these strategies fail, advanced treatment options are considered, including pallidal or subthalamic nucleus Deep Brain Stimulation (DBS) [7] or pharmacological interventions that can provide CDS [8] such as subcutaneous apomorphine infusion or levodopa/carbidopa intestinal gel (LCIG, Duodopa®) infusion [9].
Duodopa® is a novel treatment option consisting of continuous infusion of LCIG by means of a portable, patient controlled pump, directly to the duodenum through a permanent catheter implanted via Percutaneous Endoscopic Gastrojejunostomy (PEG). The principle behind Duodopa® therapy is stabilizing levodopa plasma levels by bypassing the stomach and transferring a continuous infusion of levodopa directly to the area of absorption in the small intestine, thus enhancing CDS [10,11].
Since its introduction, several studies have shown that LCIG is effective in reducing levodopa-associated motor complications, although well controlled trials with large patients numbers are still lacking. Drug related adverse reactions were similar to those reported by patients on oral levodopa, but procedure or device related complications were common, consisting mainly of intestinal tube dislocation, or occlusion, discomfort, secretion or infection in area of stoma [12,13]. LCIG is approved for clinical use in more than 40 countries and has been used in over 2,800 patients worldwide [13]. Duodopa® was approved by the Israeli Ministry of Health in May 2010 and became available for patients with complementary health insurance schemes in 2 of 4 health maintenance organizations in Israel. Application for inclusion in the 2014 health basket is now under consideration. The objective of this report is to present our initial experience with Duodopa® including feasibility, efficacy and safety of this treatment.
Methods
Patients
Consecutive patients with idiopathic Parkinson’s disease [12] referred for LCIG therapy in the Parkinson’s disease and Movement Disorders Clinic at Sheba Medical Center were included in this report. All patients had advanced PD and experienced significant motor fluctuations, prolonged and disabling daily “off” time, severe dyskinesia and non-satisfactory response to oral treatment, DBS, or both. Suitability to Duodopa® treatment was evaluated by a movement disorders neurologist and nurse, and in specific cases also by a social worker for availability of a committed caregiver as well as for the potential for general adherence to treatment.
Treatment protocol
Patients who were found suitable for LCIG treatment were hospitalized for the first treatment phase (titration phase) which included infusion of the LCIG through a naso-duodenal tube. On the first day the tube was inserted and the patient was treated with domperidone to promote the passage of the tip of the tube to the duodenum. The next morning, following discontinuation of all oral anti-PD medications and verification of the tube location by x-ray, the patient was connected to the external portable programmable pump (weighing 0.5 kg) attached to a 100 ml LCIG cassette. The initial morning dose and LCIG infusion rate were calculated based on the oral medication regimen and the patient’s regular Levodopa Equivalent Daily Dose (LEDD). After an initial morning dose the infusion was administered and dose titration was continuously managed by a dedicated Duodopa® nurse observing the patient closely. During the 3-5 days of titration phase, the optimal rate of LCIG infusion was determined, as were magnitude of the morning dose and extra doses. The infusion was discontinued every night at bedtime and restarted the next morning. During this period the patient was observed by a movement disorders neurologist that documented the motor response with a special emphasis on motor fluctuations and dyskinesia.
Patients who had a favorable response to the infusion with prolongation of motor benefit (“on” phase) and diminution of dyskinesia were referred for the second (“permanent”) treatment phase. The week after, the patients were scheduled for placement of a PEG, by a single gastroenterologist (AL) at the institute of gastroenterology. The following day patients stopped all oral antiparkinsonian therapy and LCIG infusion was initiated as the only daily antiparkinsonian treatment (if needed, nocturnal oral levodopa addition was permitted). After discharge patients were accompanied by the dedicated Duodopa® nurse that was available for phone calls or home visits as needed; Patients attended the outpatient clinic every 2-4 months for follow up visits.
Efficacy evaluation
Clinical assessments were done prior to the titration phase and following stabilization on the treatment, 2-3 months after the procedure, and included: patient and caregiver interview, Clinical Global Impression of Change (CGI-C) filled separately by the patient, caregiver and treating physician, and rating of motor deficits and complications using the Unified Parkinson’s Disease Rating Scale (UPDRS) [15]. Adverse events were documented in the patient’s file.
Results
Between January 2012 and October 2013, seventeen patients went through the titration phase. One patient did not improve with LCIG, 1 had insufficient familial support and 4 patients decided not to continue to permanent treatment. Eleven patients proceeded to PEG placement. One patient stopped the treatment shortly after placement; therefore the final analysis included 10 patients (6 males).
Three patients were relatively young and fully active that needed prolongation of their “on” time. Two patients suffered from mild to moderate dementia, needing care and supervision. Two patients had severe and disabling comorbidity and 3 patients had failed or no longer responded optimally to DBS.
The mean age was 67.3±9.5 years (range: 55-87 years), and the mean disease duration was 16.1±5.8 years (range: 7-28 years). The mean follow-up time of LCIG treatment was 11.1±8.5 months (range: 3- 22 months). Three of the patients were relatively young and fully functional, 2 had mild-moderate dementia, 3 had previous DBS operation (which was removed in one due to infection and was no longer beneficial in the other two patients) and two patients had severe comorbidity. Eight patients were treated with daytime infusions (two of which needed additional night-time oral levodopa) and 2 additional patients, suffering from severe nocturnal akinesia, required nocturnal LCIG infusion.
Eight patients consumed more levodopa (11%- 55%) following the procedure and an increment of the mean LEDD following the procedure was evident (1141± 828 mg and 1644 ± 634 mg on oral treatment and by LCIG treatment respectively).
All 10 patients reported significant reduction of daily “off” time (UPDRS item 39) as well as improvement in dyskinesia duration and disability (UPDRS items 32 and 33) (Table 1). Five patients had an improvement of at least 25% in the motor “on” UPDRS score. As evident from the CGI-C (Figure 1) all 8 patients who were able to fill the questionnaire were very satisfied with the treatment. One spouse, who was reluctant to the procedure, reported that the patient was very much worse although the patient’s and physician’s CGI reported improvement and a marked reduction of the UPDRS score was also evident. One patient (no. 4) who was severely disabled and needed 24 hours caregiver assistance regained his independence following the treatment. No change was evident in non-motor symptoms although one patient with dementia had some improvement of his confusion and became more cooperative with his caregiver.
Patient Number
Gender
Age
Disease duration
F/U
Motor UPDRS Pre
Motor UPDRS Post
LED Pre
LED Post
"off" duration Pre
"Off" duration Post
Dyskinesia duration Pre
Dyskinesia duration Post
Dyskinesia disablity Pre
Dyskinesia disablity Post
1
M
66
17
22
39
0
0
1
2
1
2
2120
3300
29
2
M
69
12
20
56
0
3
0
3
1
3
2350
2405
35
3
M
87
28
16
59
0
2
0
2
1
2
2175
1400
51
4
M
65
21
12
39
0
3
1
3
0
1
2470
1650
4
5
F
60
16
12
45
0
0
1
2
1
3
1110
1000
44
6
M
76
7
9
16
2
2
1
3
0
3
1132
1100
6
7
M
72
18
7
56
3
3
3
3
2
3
1272
675
56
8
F
55
13
6
21
1
1
1
3
1
3
1044
660
13
9
F
57
18
4
17
1
3
1
3
1
3
1028
1000
17
10
F
66
11
3
34
0
4
0
4
1
3
1320
1225
33
Age and disease duration in years, F/U: Follow-Up (months); LED: Levodopa Equivalent Dose (mg). Motor UPDRS: Motor (III) section at best "on" state. Scores for "off" duration (UPDRS item 39): 0 = none, 1 = 1-25% of the day, 2 = 26-50% of the day, 3 = 51-75% of the day, 4 = 76-100% of the day. Scores for dyskinesia duration (UPDRS item 32): 0 = none, 1 = 1-25% of the day, 2 = 26-50% of the day, 3 = 51-75% of the day, 4 = 76-100% of the day. Scores for dyskinesia disability (UPDRS item 33): 0= not disabling, 1= mildly disabling, 2 = moderately disabling, 3 = severely disabling, 4 = completely disabled.
Table 1: Laboratory values at hospital admission.Patient’s characteristics and efficacy measures of LICG treatment.
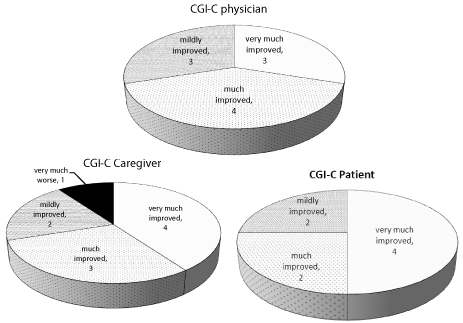
Figure 1: Clinical global impression of change scoring in PD patients treated
with LCIG.
Transient procedure or device-related adverse events occurred in 6 patients. Two had abdominal pain after the PEG placement procedure, which was due to free air in the abdomen in one and paralytic ileus in the other, both resolved with conservative treatment. Three patients had tube obstruction and one accidentally retracted the tube. All these were treated conservatively. One patient experienced worsening of her insomnia. Another patient (no. 3), an 87-year-old man, exhibited prolonged apathy following LCIG treatment and although fluctuations and dyskinesia improved he did not gain any functional improvement. He died 16 months following LCIG initiation.
Discussion
In this small study our initial experience with LCIG treatment has been positive as this treatment proved to be very effective for the motor fluctuations, dyskinesia, motor scores and global well-being of patients with advanced PD with a reasonable safety profile.
The improvement of motor performance, documented in our patients, is in accordance with previous reports showing the beneficial effect of LCIG on disease symptoms and signs [16-21] and quality of life [22,23] in patients with advanced PD and with the two recently published systemic literature reviews, [12,13] summarizing that “Clinical trials indicate that LCIG significantly improve motor complications (reduction of time in “off “ and time in “on” with dyskinesias), motor scores using the UPDR scale, non-motor symptomatology and health-related quality of life in advanced PD patients” [12].
The beneficial effect of LCIG is attributed to the CDS achieved by gastric bypass and continuous infusion directly to the area of absorption in the small intestine [6].
While there are no formal guidelines for patient’s selection or a typical profile for the ideal patient that may benefit from LCIG treatment, we have recognized in our cohort of patients with advanced and significantly disabling PD, 4 distinct groups of patients that could benefit from this treatment. The first included relatively young, fully active and generally independent patients that needed prolongation of their “on” time in order to cope with their multiple indoor and outdoor activities. The second group consisted of patients suffering from mild to moderate dementia, needing care and supervision at both “on” and ““off” states and were not candidates for DBS. The third group encompassed patients with severe and disabling comorbidity and the fourth group consisted of patients who failed or no longer responded optimally to DBS. This heterogeneity in our group demonstrates the wide spectrum of patients that can be treated with this novel treatment option. It is suggested that a clinician would consider this procedure in patients who are not candidates for DBS due to advanced age, other comorbidities or concomitant cognitive and neuropsychiatric complications.
While LCIG treatment in our group of patients was associated with relatively frequent device and procedure related adverse events, our safety data are not different from previous reports. Devos et al. [17] reported adverse events related to the infusion system in 63% of their patients and in the systematic literature review technical problems with the infusion device have occurred in up to 70% of patients [12]. It is important to note, however, that all the adverse events were not severe or life threatening and could be successfully treated conservatively.
Conclusion
LCIG via PEG is a novel and promising treatment option that can dramatically improve motor performance and quality of life in patients with advanced PD.
References
- de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006; 5: 525-535.
- Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, et al. Levodopa in the treatment of Parkinson's disease: current controversies. Mov Disord. 2004; 19: 997-1005.
- García-Ruiz PJ, Del Val J, Fernández IM, Herranz A. What factors influence motor complications in Parkinson disease? a 10-year prospective study. Clin Neuropharmacol. 2012; 35: 1-5.
- Nyholm D. The rationale for continuous dopaminergic stimulation in advanced Parkinson's disease. Parkinsonism Relat Disord. 2007; 13: S13-17.
- Chase TN. The significance of continuous dopaminergic stimulation in the treatment of Parkinson's disease. Drugs. 1998; 55: 1-9.
- Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. The "on-off" phenomenon in Parkinson's disease. Relation to levodopa absorption and transport. N Engl J Med. 1984; 310: 483-488.
- Israel Z, Hassin-Baer S. Subthalamic stimulation for Parkinson's disease. Isr Med Assoc J. 2005; 7: 458-463.
- Schwartz M, Sabetay S. An approach to the continuous dopaminergic stimulation in Parkinson's disease. Isr Med Assoc J. 2012; 14: 175-179.
- Antonini A, Tolosa E. Apomorphine and levodopa infusion therapies for advanced Parkinson's disease: selection criteria and patient management. Expert Rev Neurother. 2009; 9: 859-867.
- Othman AA, Dutta S. Population pharmacokinetics of levodopa in subjects with advanced Parkinson's disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br J Clin Pharmacol. 2014; 78: 94-105.
- Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson's disease patients. AAPS J. 2013; 15: 316-323.
- Fernandez HH, Odin P. Levodopa-carbidopa intestinal gel for treatment of advanced Parkinson's disease. Curr Med Res Opin. 2011; 27: 907-919.
- Nyholm D. Duodopa® treatment for advanced Parkinson's disease: a review of efficacy and safety. Parkinsonism Relat Disord. 2012; 18: 916-929.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55: 181-184.
- Fahn S, Elton R, Members of the UPDRS Development Committee. Fahn S, Marsden CD, Calne DB, Goldstein M, editors. In: Recent Developments in Parkinson’s Disease. Florham Park, NJ. Macmillan Health Care Information. 1987; 153-163, 293-304.
- Antonini A, Isaias IU, Canesi M, Zibetti M, Mancini F, Manfredi L, et al. Duodenal levodopa infusion for advanced Parkinson's disease: 12-month treatment outcome. Mov Disord. 2007; 22: 1145-1149.
- Devos D. French Duodopa study group. Patient profile, indications, efficacy and safety of duodenal levodopa infusion in advanced Parkinson’s disease. Mov Disord. 2009; 24: 993-1000.
- Eggert K, Schrader C, Hahn M, Stamelou M, Rüssmann A, Dengler R, et al. Continuous jejunal levodopa infusion in patients with advanced parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol. 2008; 31: 151-166.
- Fernandez HH, Vanagunas A, Odin P, Espay AJ, Hauser RA, Standaert DG, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson's disease open-label study: interim results. Parkinsonism Relat Disord. 2013; 19: 339-345.
- Olanow CW, Keiburtz K, Odin P Alberto J Espay, David G Standaert, Hubert H, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomized, controlled, double-blind, double-dummy study. Lancet Neurol. 2014; 13: 141-149.
- Zibetti M, Merola A, Artusi CA, Rizzi L, Angrisano S, Reggio D, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson's disease: a 7-year experience. Eur J Neurol. 2014; 21: 312-318.
- Antonini A, Mancini F, Canesi M, Zangaglia R, Isaias IU, Manfredi L, et al. Duodenal levodopa infusion improves quality of life in advanced Parkinson's disease. Neurodegener Dis. 2008; 5: 244-246.
- Honig H, Antonini A, Martinez-Martin P, Forgacs I, Faye GC, Fox T, et al. Intrajejunal levodopa infusion in Parkinson's disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord. 2009; 24: 1468-1474.