Abstract
Dopamine along with other chemical messengers like serotonin, cannabinoids, endorphins and glutamine, play significant roles in brain reward processing. There is a devastating opiate/opioid epidemic in the United States. According to the Centers for Disease Control and Prevention (CDC), at least 127 people, young and old, are dying every day due to narcotic overdose and alarmingly heroin overdose is on the rise. The Food and Drug Administration (FDA) has approved some Medication-Assisted Treatments (MATs) for alcoholism, opiate and nicotine dependence, but nothing for psychostimulant and cannabis abuse. While these pharmaceuticals are essential for the shortterm induction of “psychological extinction,” in the long-term caution is necessary because their use favors blocking dopaminergic function indispensable for achieving normal satisfaction in life. The two institutions devoted to alcoholism and drug dependence (NIAAA & NIDA) realize that MATs are not optimal and continue to seek better treatment options. We review, herein, the history of the development of a glutaminergic-dopaminergic optimization complex called KB220 to provide for the possible eventual balancing of the brain reward system and the induction of “dopamine homeostasis.” This complex may provide substantial clinical benefit to the victims of Reward Deficiency Syndrome (RDS) and assist in recovery from iatrogenically induced addiction to unwanted opiates/ opioids and other addictive behaviors.
Keywords: Dopamine; Glutamine; Enkephalin; Medication Assisted Treatment (MAT); KB220Z; Reward Deficiency Syndrome (RDS)
Introduction
The big question is - “what is the best way, based on scientific evidence, to provide a balanced brain in people involved in addiction treatment and recovery”? While the answer may not be simple, because of the enormous efforts made by our national institutes (NIAAA and NIDA) we are making progress. The fifty-year journey began in the late 60’s and 70’s with the investigation of neurotransmitters in 1969 [1,2] that revealed that dopamine could control tremors in the periphery of cats (Figure 1).
Figure 1: Dopamine chemical structure. The chemical structure of the
compound Dopamine the happiness molecule is C8H11NO2. Dopamine
affects the brain processes that control emotional responses and ability to
experience pleasure, desire and motivation.
Understanding the neurochemistry of addiction
In 1968, Blum and Geller received a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to do animal research into the role of neurotransmitters in stress and aberrant alcohol drinking. Funded by this grant, their laboratory was the first to look into darkness induced drinking based on the effect of pineal gland melatonin [2]. They discovered that drinking was increased because there was an increase in the synthesis of melatonin in darkness which is inversely proportional to a reduction in serotonin. In fact, injecting melatonin during the light phase also induced high alcohol intake [2]. From 1968-1972, the research focused on the role of stress-induced changes in brain neurochemistry. One finding that influenced the development of KB220 was the discovery that reduced serotonin in the brain of rodents resulted in intense stress-related behavior [3].
In 1972, at the Department of Pharmacology, University of Texas Health Science Center, at San Antonio, Texas (UTHSCSA), Blum’s group continued research on alcoholism. During this time, the concept of shared neurochemical mechanisms between alcohol and opiates [4] was developed and presented to the scientific community. The research was the first to show that the narcotic antagonist naloxone could, not only, block alcohol induced sleep-time in mice (Figure 2), but that naloxone could also block alcohol dependence [5]. These controversial early findings led to the clinical development of Vivitrol (Naltrexone) and Suboxone/Zubsolve (buprenorphine/ naloxone) used currently as FDA approved pharmaceuticals to treat both alcoholism as well as opiate addiction [6]. Along these lines, it was shown that both dopamine and morphine could reduce alcohol withdrawal symptoms in a similar fashion [7,8].
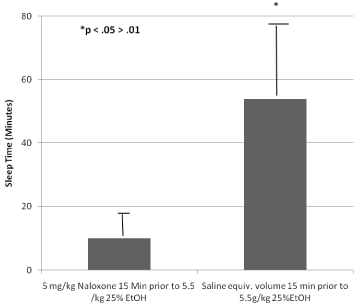
Figure 2: Naloxone Suppression of Ethanol Narcosis. The first experimental
evidence to show that opiates and alcohol have Common Mechanisms with
permission [12].
Blum’s group turned their attention to evaluating the role of Isoquinolones (a by-product of the combination of the acid form of alcohol and dopamine) in alcoholism. Work in the early 70’s by Houston and New York scientists [9,10] suggested that when one drinks alcohol a substance Isoquinolones (Salsolinol) is formed in the brain which resembles the opiates found in poppy plants (Figure 3).
Figure 3: Chemical structure of Isoquinolone Salsolinol. Isoquinolone
Salsolinol is involved in condensation of dopamine and acetaldehyde
influences alcohol intake in humans and animal models.
The next five years were devoted to the exploration of the possibility of a neurochemical commonality between two seemingly very diverse chemicals, alcohol and morphine. During this time, an isoquinolone metabolite was identified in the brain of mice following ethanol ingestion [11]. This finding led to the idea of a shared brain mechanism that occurs in both alcohol and opiate addiction. The mechanism was described in an edited book on the subject published in 1978 [12]. This work was important because it set the stage for understanding the basis for cross addictions or poly-drug abuse (Figure 2). The unconventional idea that the “junkie” in the street looking for a heroin fix is neurochemically similar to the executive drinking five martinis for lunch.
Addiction science was advanced following the seminal discovery of the opiate receptor published in Science [13] in 1973 (Figure 4). The discovery of endogenous brain peptides followed. Endogenous meaning these peptides occur naturally within the body, eventually called “Endorphins” a term first coined by Eric Simon [14]. Research began dedicated to finding the function of Endorphins. During the late 70’s and early 80’s, most scientists did not believe that the Endorphins (brain chemicals that act like morphine) had anything to do with the effects of alcohol. This finding, however, became critical factor in the actual development of KB220.
Figure 4: Proposed Opiate Receptor. One of many images of the opiate-type
receptor.
The very first finding that showed the unequivocal role of one endorphin-like brain substance called methionine-enkephalin (METENK) was published in the prestigious journal Proceedings of National Academy of Science (PNAS) [15]. The study demonstrated that alcohol intake in different genetically bred mice (they love or hate alcohol) was in proportion to brains content of METENK. Specifically, low METENK caused high alcohol drinking, while, high METENK induced low alcohol intake (Figure 5). Concurrently, the concept that genes might be one reason why people cannot control their drinking was developed. Could genes be, in part, responsible for low levels of endorphins (morphine peptides)? One solution to this unresolved problem would be to find a way to overcome low endorphin levels.
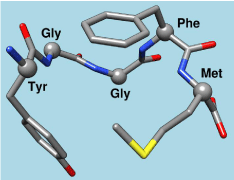
Figure 5: Chemical Structure of Met-Enkephalin. One of many images of the
natural brain opiate methanine enkephalin as a peptide.
The question of whether the problem was environmental, as well as, genetic was asked. In 1982, Blum et al. published seminal work in Science [16]. The experiment involved alcohol loving Golden Syrian hamsters. After drinking alcohol freely for one year (the equivalent to 20 years in humans) a very significant reduction of leucineenkephalin synthesis (production) in the striatum (a brain region involved in craving behavior) was demonstrated (Figure 6).
Figure 6: Drinking Alcohol Destroys Natural Brain Opioids. Experiments
showing the reduction of synthesis of leucine-enkephalin following one year
chronic alcohol drinking in golden Syrian hamsters approximates 20 human
years [16] with permission.
This finding has since then, been confirmed in humans and shown by others to occur with chronic intake of opiates, diazepam and cocaine. Most recently, Margolis et al. [17] reconfirmed these earlier findings. The conclusion that enkephalin synthesis reduction is a common effect of various psychoactive substances lent support to the idea that all addictions might well share brain mechanisms (in this case low enkephalins). At that time Blum et al. were the first to show that intra-cerebellum injections of enkephalamide (a methyl analog of enkephalin that prevents the quick breakdown of the substance) induced a significant reduction of alcohol intake in C57/blk mice genetically bred to be high ethanol seeking. Together, these studies pointed to the idea that low endorphins in the brain lead to high alcohol drinking. Essentially, one way to prevent excessive alcohol intake could be to find a way to increase brain levels of endorphins [18].
Following these important findings, Blum’s group was the first to report on the concept of “pharmacogenomic engineering” accomplished by using a substance known to stop an enzyme carboxyl- peptidase. The function of the enzyme enkephalinase is to slice up the five amino acids string that makes up enkephalin. D-phenylalanine is the substance used to block the destruction of enkephalin by blocking the biological activity of enkephalinase in the brain. D-phenylalanine was a good candidate because it was touted in the 1930’s as an inhibitor of the enzyme carboxyl- peptidase, at the time having nothing to do with brain opioids or brain opium. Understanding this and based on earlier work by others [19,20] D-Phenylalanine was evaluated as a potential anti-alcohol agent (Figure 7).
Figure 7: Cartoon showing that raising brain endorphines with the known
enkephalinase inhibitor D-Phenyalanine metabolite Hydrocinnamic Acid
blocks alcohol craving. Blum et al. with permission unpublished.
Administration of D-Phenylalanine for 18 days in alcohol loving c57/blk mice showed that this substance raised endorphin levels in both the pituitary and the striatum. Thus, mice that were genetically prone to seek alcohol reduced their alcohol intake so much that the new levels were similar to the levels of non-preferring (alcohol hating) DBA mice. This work published in the journal Alcohol [20] provided the starting point for the role of enkephalinase inhibition as a therapeutic agent for the treatment of alcoholism. Until now little effort has been made by the pharmaceutical companies to develop enkephalinase inhibition as a viable anti-alcohol therapeutic agent.
In 1982, we began to develop KB220. Realizing that an antialcoholic agent must constitute a number of select precursor neurotransmitter based amino-acids; an enkephalinase inhibitor and inhibitors of both mitochondrial and synaptic enzyme catabolizes (destroyers) of brain chemical messengers such as serotonin and dopamine. In essence, D-phenylalanine fooled the enkephalinase enzyme by having a high affinity whereby the enzyme bound preferentially to the D-amino acid instead of methionine-enkephalin at the glycine-phenylalanine binding site (Figure 5).
The first “neuronutrient” formulations
Following many attempts the first ever “neuronutrient” was formulated and resulted in a successful reduction of heavy drinking in a male alcoholic. Further reiterations of the neuronutrient formula also resulted in the prevention of serious drinking in a female alcoholic. The first commercialization of this neuronutrient KB220 was utilized in over 1,000 treatment centers in the USA during the next eight years. Development of formulations for cocaine dependence, opiate dependence and even obesity was continued [21,22] (Table 1). The FDA released information that the l-amino-acid tryptophan caused 35 deaths due to eosinophilia and withdrew L-Tryptophan from the consumer marketplace. The FDA later rescinded the withdrawal of L-Tryptophan.
GRAS NUTRIENT
PATHWAY
D-Phenylalanine
Opioid peptides
L-Phenylalanine
Dopamine
L-Tryptophan
Serotonin
L-Tyrosine
Dopamine
L-Glutamine
GABA
Chromium
Serotonin
Rhodiola rosea
COMT
N-acetyl-cysteine
Glutamine
Pyridoxine
Enzyme catalyst
Table 1: KB220 Ingredients [40] with permission.
Brain reward cascade
This research continued, Blum and Gerald Kozlowski of Southwestern Medical School in Dallas, reviewed many scientifically sound research articles [23]. They began to track the various ways the neurotransmitters interact within the brain. This fundamental research resulted in an understanding represented on a detailed map. Now well-known, the reward circuitry of the brain was first described by the term “Brain Reward Cascade “(BRC) and published in 1989. Many investigations by global scientists, [24] over the last 25 years have supported this basic conceptual framework. This idea has stood the test of time with recent modifications and is used as a blue-print of neurotransmitter interaction and subsequent workings of the reward system and reward-dependent behaviors (Figure 8).
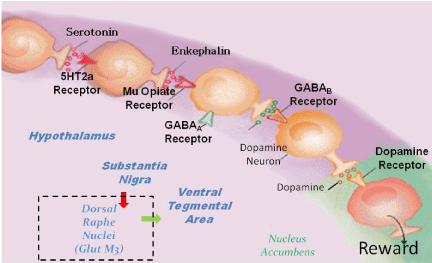
Figure 8: Brain Reward Cascade. Schematic of the brain reward cascade
showing how dopamine is released at the Nucleus Accumbens [29] with
permission.
While somewhat controversial, the basic tenant of this work revealed that the feeling of “well-being” can only be achieved when the molecule dopamine is released in the nucleus accumbens at balanced “homeostatic” levels. Any deviation causes “dopamine resistance” and as such can result in aberrant cravings. Specifically, too much dopamine can lead to schizophrenia and too little dopamine could lead to depression or unhappiness and craving [25] (Figure 9).
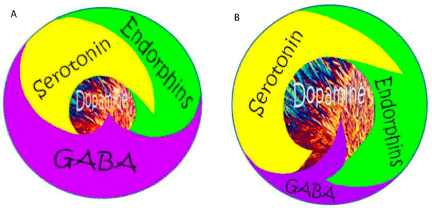
Figure 9: Schematic representation of Brain Reward Cascade.
(A) Abnormal unbalanced neurotransmission showing high GABA
transmission with reduced dopamine release: Unhappy Brain. (B) Normal
balanced neurotransmission showing appropriate amount of dopamine
release: Happy Brain feeling of well-being [38] with permission.
During this period, many research papers related to clinical outcomes including double-blind-placebo investigations appeared in the scientific literature. The response from the recovery community was very positive and as such amino-acid therapy for the treatment of drug addiction was born. These developments are detailed in an award-winning book “Alcohol and the Addictive Brain” [26].
The genetic association
Following this research, Blum’s group began investigating the well-known theory that alcoholism is a familiar disorder. Specific studies that related any gene (s) with this inheritable global problem were lacking. Blum and Ernest P. Noble, the former director of the NIAAA and Psychiatry Professor at UCLA, began to analyze brain tissue from alcoholics (80% cirrhosis of the liver) and non-alcoholics looking for genes that affect the Brain Reward Cascade. “Restriction Fragment Length Polymorphism (RFLP) techniques were used to discover the first ever genetic association with alcoholism the novel findings were published in JAMA in 1990 [27]. The now famous genetic association of the dopamine-D2-receptor gene (DRD2) A1 allele (variant) with severe alcoholism. Initially, this astounding discovery was met with controversy worldwide. Now this discovery is considered a breakthrough in addiction medicine and has been researched in almost 4,185 scientific studies cited in PUBMED (8- 27-16). In fact, the JAMA study sparked the entire field known as “Psychiatric Genetics.” Noble, Blum and associates also published that carriers of the DRD2 A1 allele are born with a 30-40% lower number of D2 receptors and are at high risk for all addictive behaviors (both substance and non-substance-related) [28].
Currently, the DRD2 gene is considered to have a substantial impact on all reward behaviors and as such has been considered a “reward gene” as first stated in our JAMA paper. Immediately after announcing the association of the DRD2 with severe alcoholism a Gallup poll revealed that the majority of Americans now believed that alcoholism is genetically based and not simply a moral characteristic (Figure 10).
Figure 10: DRD2 gene: Taq 1 A2 allele (normal association) and Taq 1 A1
allele (alcohol association) [27] with permission.
Reward deficiency syndrome (RDS)
In 1995, understanding the nature of common neurogenetic and neurobiological mechanisms shared across the major abusable licit and illicit drugs and the importance of a hypodopaminergic trait/state Blum’s group conceived and coined the term “Reward Deficiency Syndrome”(RDS).RDS describes a group of addictive, compulsive and impulsive disorders including alcoholism, attention deficit disorder, drug abuse and food bingeing, pathological gaming, internet addiction, sex addiction, having a common genetic basis acting as an umbrella term [29,30] (Table 2).
ADDICTIVE BEHAVIORS
IMPULSIVE BEHAVIORS
OBSESSIVE COMPULSIVE BEHAVIORS
PERSONALITY
DISORDERS
Substance Related
Non Substance Related
Spectrum Disorders
Disruptive Impulsive
Alcohol
Thrill seeking (novelty)
Attention-deficit Hyperactivity
Anti-social
Body
Dysmorphic
Paranoid
Cannabis
Sexual
Sadism
Tourettes and Tic Syndrome
Conduct
Hoarding
Schizoid
Opioids
Sexual Masochism
Autism
Intermittent Explosive
Trichotillo-mania
(hair pulling)
Borderline
Sedatives/
Hypnotics
Hypersexual
Oppositional Defiant
Excoriation
(skin picking)
Schizotypal
Stimulants
Gambling
Exhibitionistic
Non-suicidal
Self-Injury
Histrionic
Tobacco
Internet
Gaming
Narcissistic
Glucose
Avoidant
Food
Dependant
Table 2: Reward deficiency syndrome, a dysfunction of the reward genes [59] with permission.
One important outcome of this work was a paper by Blum et al., published in the Royal Society of Medicine showing that carriers of the DRD2 A1 allele variant have a 74.4% predictable risk for RDS even at birth [31]. This does not mean an individual is doomed because of faulty genes because the environment (through epigenetic) has at least a 50% chance of preventing the expression of these risky gene variations.
In unpublished work Andrew Smolen and Brett Haberstick (from the Institute of Behavioral Genetics, Colorado University, Boulder) and Blum developed a carefully designed ten-gene polymorphic panel of reward genes. The panel significantly predicts the Addiction Severity Index Media Version (ASI-MV) alcohol and drug severity scores. The patients were from seven different addiction-treatmentcenters in the United Sates. This genetic test can predict genetic risk for the development of addiction (necessary for pain clinics) and satisfy genetic risk for those in recovery [32].
The RDS concept was first described in a general article in the American Scientist and today over 552 articles are listed in PUBMED (4-16-16) that deal with “Reward Deficiency” and another 1014 articles deal with “Dopamine Dysregulation.” RDS is currently found and defined in MS-Word and will be included in SAGE Encyclopedia of Abnormal Psychology and Mental Illness (2017). While there is a body of literature that addresses the importance of dissecting the role of dopamine into “wanting” and “ liking,” these concepts dovetail onto the RDS model [33]. The basic concept was adopted in the ASAM new definition of Addiction in 2011. This concept expanded on the “dopamine depletion hypothesis” for cocaine abuse proposed by Dackis and Gold from the NY Psychiatric Institute and Florida University [34].
The work of Mark Gold pioneered many basic and clinical concepts that are incorporated in addiction medicine today. Gold’s group developed concepts related to the clinical utilization of naloxone in the treatment of addiction, provided the mechanism involved in opiate withdrawal and the use of clonidine and suggested that agonistic dopamine therapy should be a frontline agent in preventing cocaine dependence and relapse. The extension and acceptance of our RDS concept by Gold, Hoebel and Avena from the late 90s until the present who have proposed similar neurochemical mechanisms in both food and drugs addictions. In fact, although there is still controversy, their work and writings lend neuroscientific support to the fact that food can be addicting just like opiates [35]. Also Gold’s, recent work on second-hand smoke has paved the way for smoke-free zones across the United States [36]. Most recently a series of important papers on drug and food addiction sharing common mechanisms and reviews dealing with vaccines, gene therapy and most importantly pro-dopaminergic regulation therapy for all RDS behaviors have been published [37]. Since the 80’s until the present time over 32 peer reviewed articles showing clinical benefits of KB220 variants especially craving behavior (Figure 11) have been published.
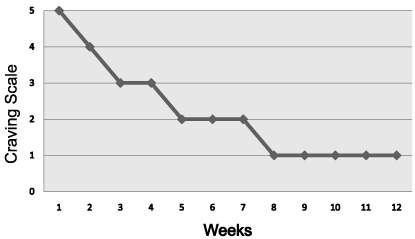
Figure 11: KB220 Reduces Craving Behavior in cocaine abusers [22] with
permission.
Functional connectivity
Recent work has been focused on the utilization of neuroimaging tools. Our group published a series of articles showing significant regulation in the pre-frontal cortices particularly in the cingulate gyrus (a region for drug relapse) in abstinent psychostimulant abusers [38,39] (Figure 12), alcoholics and opiate addicts [40]. These studies used qEEG analysis following both intravenous and /or oral administration of KB220 and also in ADHD using sophisticated qEEG software called LORETA [41].
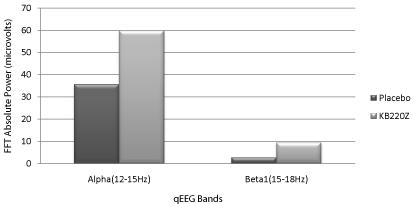
Figure 12: KB220Z Vs. qEEG Placebo in abstinent Psychostimulant Addicts.
Alpha and beta1 changes in the direction that induces calming [39] with
permission.
Recently neuroimaging studies have uncovered reduced resting state functional connectivity (rsFC) as a culprit in addictive behaviors. “Normal” rsFC can be understood as “cross talk” meaning that different parts of the brain communicate, for example, the hippocampus (memory) talks with the accumbens (craving) talks with the Cingulated Gyrus (decision-making). Reduced functional connectivity at rest puts the individual at risk for addictive-like behaviors -view the cartoon of Swiss cheese (Figure 13).
Figure 13: Functioning brain connections represented by cheese. Addictive
Brain: lacks connectivity at rest represented by holes (no cross –talk)
compared to Non-Addictive Brain. KB220Z helps restore resting state
functional connectivity and consequentially better decision making.
At rest, the brain of an individual with a normal genetic trait, or epigenetic (environmental) state, has background working connections, which is a good thing illustrated in the figure as American cheese (Figure 13). Certainly, it is now known that drug addiction and other non-drug addictive and RDS behaviors, like gambling, compulsive sexual behavior, overeating and ADHD all reduce rsFC [42-51]. These changes are thought to be epigenetic. The work of Eric J Nestler and others forged a real path to understanding how the environment influences DNA gene expression [52].
In unpublished work with non- addicted rats, KB220Z was shown to activate connectivity when compared to a placebo. The regions of the brain that are activated include the areas that are used for memory, decision-making and craving. These areas include the prelimbic and infralimbic loci, the nucleus accumbens, the cingulate gyrus, anterior thalamic nuclei and the hippocampus (Figure 14). Just as epigenetic changes occur due to drug toxicity and stress to reduce (like reduced rsFC), positive epigenetic changes (like increases in rsFC) may occur due to KB220.
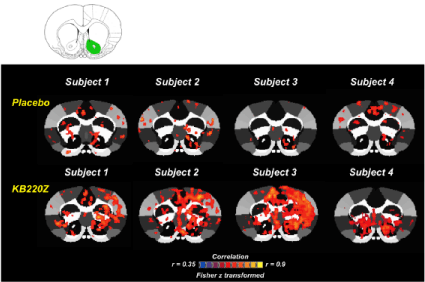
Figure 14: KB220Z and rsfMRI in N. Accumbens. Unpublished Febo and
Blum with showing bold dopamine activation 5-15 minutes following KB220Z,
with permission.
The foremost finding published to date concerning KB220Z (a glucoside variant) shows that in a placebo-controlled crossover study, rsFC was significantly restored, in abstinent heroin addicts. In addition, one-hour post oral KB220Z, dopaminergic pathways were activated, heightened emotionality seen as hyper dopaminergic activity in the putamen is reduced and rsFC is restored [53] (Figure 15). The rsFC was restored across a network that included the cerebellum, posterior cingulate, nucleus accumbens, medial frontal gyrus, occipital cortical areas and the dorsal anterior cingulate where rsFC is impaired due to withdrawal from heroin. Decision-making deficits persist in protracted abstinence from stimulant and opiate addiction and improvements in decision-making takes years [54-55]. The findings regarding the dopamine homeostatic effect of KB220Z in this study clearly represent a possible breakthrough in the treatment of cognative dysfunction during recovery.
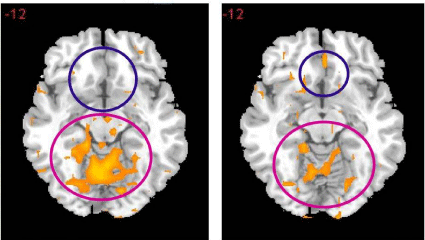
Figure 15: Resting-State fMRI 1 hour after one dose KB220Z. Placaebo Left
side. Vs. KB220Z Right side [53] with permission.
Recovery and the abstinence model
Many people do well in recovery, lifting both their spirituality and fellowship by attending 12 step type programs like Alcoholics Anonymous (AA) and Narcotics Anonymous (NA). This success can be understood in terms of “The molecular neurobiology of the 12 steps” described in detail in the Springer Neuroscience Brief publication [56]. The success of 12 steps may also be considered epigenetic (environmental).
The federal agency responsible for most of the public funding for drug addiction treatment the Substance Abuse and Mental Health Services Administration (SAMHSA) has for the first time, added language to its grant applications recommends that the treatment industry should move away from the abstinence model [57]. From the beginning1935 until the present AA, has promoted the abstinence model; the idea of not using any substances including medications prescribed specifically for addiction, is the only acceptable route to recovery.
SAMHSA’s new initiative driven by block grant applications for fiscal years 2016-2017 appears to require the medication-assisted treatment (MAT) option in clinical settings. SAMHSA’s grants in the fiscal year 2015 involved $1.8 billion. While it is generally agreed that FDA approved MAT including buprenorphine/naloxone combinations for opioid addicts, may be useful for short-term treatment the vast majority of rehabilitation facilities in the U.S. do not offer such care. In addition, FDA approval is limited to MAT for Opioid, Alcohol and Nicotine Dependence,
However there are no medications approved for Cocaine and Marijuana abuse. Even NIDA and NIAAA realize that MAT is not optimal and continue their efforts to find better treatments.
The US, the heroin and prescription opioid epidemic
In the US, the heroin and prescription opioid epidemic targets and kills our kids and future generations (see film “Kids Are Dying”) [58]. The Centers for Disease Control and Prevention reported that heroin-related overdose deaths almost quadrupled between 2002 and 2013. In response, Michael Botticelli federal drug czar stated that Federal Drug Court funding would be made conditional, based on states being guided by the science of treatment, rather than ideology. Certainly moving in this direction would pave the way for even better long-term beneficial treatments.
The currently FDA-approved drugs are predominantly dopamine antagonists that favor blocking dopamine [59]. Although Zubsolve/ Suboxone buprenorphine in buprenorphine/naloxone combinations (like Suboxone) is a partial mu opiate receptor agonist, following longterm use, it has been shown to lead to typical withdrawal symptoms and in some cases even suicide ideation [60]. When long-term treatment with buprenorphine/naloxone combinations was compared with short-term treatment, the analysis revealed no significant benefit from long-term vs. short-term treatment and no improvement in clinical outcomes [32,61]. Functional MRI studies reveal almost 98% saturation of Mu opiate receptors with 16mg of Buprenorphine signifying that dose escalation of buprenorphine/ naloxone is neither practical or beneficial.
For many decades, investigators have accepted the tenant that “Dopamine Function” within normal parameters is a cornerstone of a healthy and happy life (Figure 9). Thus, except to achieve acute “Psychological Extinction” which is produced when a particular addictive behavior is no longer reinforced by the consequences “high” and the behavior gradually stops occurring. Thus, except to achieve acute “Psychological Extinction” it makes little sense to block dopamine’s activity in the long-term [59]. Indeed a problem with this approach is substitution of drug of choice, when, for example, food addictions are treated with bariatric surgery and the individual moves on to alcohol or a different non drug addiction like gambling or sex to provide the dopamine deficit. In fact, we have shown that the long-term use of buprenorphine/naloxone combinations-induce a flattening affect in one’s normal personality when compared to the general population and AA groups [62].
So while it is important to embrace short-term dopamine antagonistic therapy as espoused by FDA approval of MAT drugs, we do not recommend them for long-term use. Understandably, while many of the proponents of current MAT would argue against this premise, we are looking for ways to normalize dopamine regulation.
In the mid-80’s Mark Gold’s group was on the right track when they proposed the use of Bromocriptine, a potent D2 agonist, to treat cocaine addiction [63]. However, this idea did not hold up because when Bromocriptine was used on a chronic basis, it caused a reduction in the numbers of D2 receptors [64]. This idea however along with earlier work suggesting that “dopamine agonist therapy” and not “dopamine antagonist therapy” should be embraced in the long-term treatment of addictions seemed reasonable. Individuals displaying RDS behaviors (addictive) have been shown to possess low dopaminergic function, due to stress and the toxic effect of substances (epigenetic) and/or genetics [65]. A number of gene variants called polymorphisms that associate with addiction risk have been identified. They are variations of the reward genes that are responsible for genetic functions that effect synaptic dopamine availability. They include polymorphisms of the DRD1-4; DAT1; Serotonin transporter, GABA, Mu-Opiate receptor, COMT, MAO genes and victims of RDS may have varying numbers of these polymorphisms [66].
Can you imagine if we could provide regulation or “normalization” of dopaminergic function leading to what has been termed “dopamine homeostasis?” In this way, whether there is either an acute “hyperdopaminergic” or chronic hypodopaminergic trait or state [67], it is the balancing of the brain’s neurotransmitter signaling that should work best. In fact, only a very small percentage of treatment centers currently embrace this concept by offering dopamineboosting modalities such as Yoga Meditation, Yoga Exercise, Brain Spotting, Behavioral Cognitive Therapy, Trauma Therapy, Sound Therapy, Music Therapy and the serving up of “dopamine for dinner” [68]. The literature in some cases like, Yoga and Mediation shows a 65% increase in neuronal dopamine [69]. Moreover, certain healthy low glycemic foodstuffs, like fish oils, are known to boost dopamine function. However, due to lack of research, little direct evidence for dopamine boost has been linked to other holistic approaches.
Vulnerability to relapse
Regarding vulnerability to relapse there is evidence that carriers of the A1 form of the DRD2 gene have an increased risk for relapse [70] and if they enter treatment a high chance that they will leave against medical advice (AMA). However, research has shown a significant reduction in not only AMA (Figure 16) rates but reduce relapse (Figure 17) with the use of KB220Z.
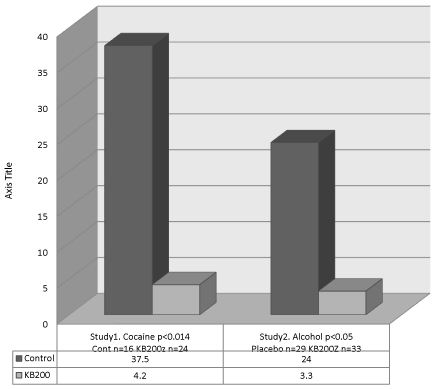
Figure 16: AMA rate without vs. with KB220 variant. Study 1 [21] and Study
2 [38] with permission.
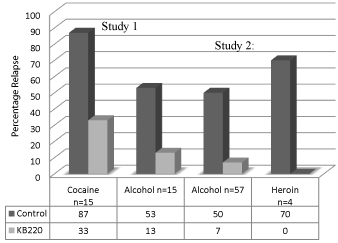
Figure 17: Relapse without vs. with KB220 variant. Study 1. Brown et al.
Journal of Psychoactive Drugs 22:173-187 (1990) after 10 months (p<0.001)
[22] and Study 2. Chen et al. Adv. Therp. 24: 402-414 (2007) after 12 months
(p<0.001) [74].
Both compliance to FD Approved MAT and abstinence from drugs of abuse in urine drug screening data from the Comprehensive Analysis of Reported Drugs (CARD)™ from patients attending chemical dependency programs in six states on the east coast of America were analyzed [71]. The take-home message is that while MAT is presently indispensable, it brings about psychological extinction, the study found that at the end of inpatient treatment overall compliance was 78.4 percent and abstinence 57.3 percent in these patients. This disappointing result indicates that better treatment strategies are required for recovery from addiction. Enhancing dopamine function an alternative to dopamine blockade in long term may be one such strategy [71].
Along these lines “Glutaminergic-Dopaminergic Optimization Complex Therapy” known as KB220, has been well-researched in many clinical trials. KB220z has been shown to provide gentle activation of dopamine across the brain reward circuitry in abstinent heroin (Figure 15) and abstinent psychostimulant addicts (Figure 15). Additionally, significant increases in resting state functional connectivity have been demonstrated in animal models using state of the art resting state fMRI measurements (Figure 14). Continued required research may result in showing that long-term dopamine agonist therapy with a KB220 variant leads to needed “dopamine homeostasis” the missing piece in all RDS behaviors both substance and non-substance addictions.
Summary
This summary of the research results provides solid scientific support based on almost 40 years of research into Neuro adaptogen Amino-Acid Therapy (KB220). Indeed, the induction of increased rsFC and physiological changes in the brain neuroplasticity were recently demonstrated [72,73].
Future Perspective
Now the first ever aqua-nano delivery system for KB220Z has been developed. Also, there are millions of recovering addicts here in the United States of America. It is also well-known that the general non-addicted population may also have low dopamine function because of either stress or overindulgence of psychoactive drugs and associated toxicity in a non-addicted social context. Low dopamine (hypodopaminergia) has been associated with many conditions including:
- cognition (164 studies)
- reduced memory (199 studies)
- reduced decision making (45 studies)
- reduced energy (95 studies)
- impaired exercise (73 studies)
- excessive cravings (241 studies)
- physical performance (291 studies)
- aging (218 studies)
- stress (374 studies)
- sadness (257 studies)
- poor relationships(36 studies)
- lack of well-being (984 studies)
- overeating (162 studies)
Reward deficiency system solutions
At this moment, recommendations for evidenced based treatment that include: testing for genetic risk severity using the Genetic Addiction Risk Score and using the newly developed Reward Deficiency Syndrome Questionnaire to identify those at risk for RDS behaviors particularly before beginning any opioid therapy. During treatment and recovery randomized urine testing for both Compliance to MAT and Abstinence from psychoactive drug abuse should be included. Long-term dopamine agonist therapy with KB220Z to induce dopamine homeostasis is recommended for all RDS behaviors. Regarding the future, polymorphic DNA-directed mRNA genetic expression profiling might be a useful outcome measure.
Conclusion
The greatest minds in the addiction space are encouraged here to acknowledge that for the betterment of humankind finding ways to boost (regulate) not block dopamine function in the long term could prevent relapse and provide a much better life for those in recovery. The challenge to the scientific and clinical community is to admit that addiction treatment in America is broken and needs to be fixed. This admission in no way negates the daily struggle of clicians and the enormous effort of countless people who have unselfishly given so much to the field.
The FDAs push for MATS and the use of off-label Gabapentin, Topiramate and other drugs to alter the brain reward circuitry to induce cessation to the use of drug of choice is understood. Although it is indeed counter-intuitive, these pharmaceuticals have the effect of reducing needed dopamine in the long-term, resulting in flattened affect, depression and suicide ideation.
The recent restriction by the CDC of prescription opioid use in acute pain conditions is on the right track. Continued research is needed regarding the potential for return of well-being in recovery by the gentle induction of “dopamine homeostasis;” balancing serotonergic, endorphinergic, cannabinergic, glutaminergic, dopaminergic mechanisms and restoring healthy brain function and connectivity.
References
- Blum K. The effect of dopamine and other catecholamines on neuromuscular transmission. Arch Int Pharmacodyn Ther. 1969; 181: 297-306.
- Reiter RJ, Blum K, Wallace JE, Merritt JH. Effect of the pineal gland on alcohol consumption by congenitally blind male rats. Q J Stud Alcohol. 1973; 34: 937-939.
- Geller I, Blum K. The effects of 5-HTP on para-Chlorophenylalanine (p-CPA) attenuation of "conflict" behavior. Eur J Pharmacol. 1970; 9: 319-324.
- Blum K, Eubanks JD, Wiggins B, Wallace JE. Morphine withdrawal reactions in male and female mice. Am J Drug Alcohol Abuse. 1976; 3: 363-368.
- Blum K, Futterman S, Wallace JE, Schwertner HA. Naloxone-induced inhibition of ethanol dependence in mice. Nature. 1977; 265: 49-51.
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370: 2063-2066.
- Blum K, Eubanks JD, Wallace JE, Schwertner HA. Suppression of ethanol withdrawal by dopamine. Experientia. 1976; 32: 493-495.
- Blum K, Wallace JE, Schwerter HA, Eubanks JD. Morphine suppression of ethanol withdrawal in mice. Experientia. 1976; 32: 79-82.
- Cohen G, Collins M. Alkaloids from catecholamines in adrenal tissue: possible role in alcoholism. Science. 1970; 167: 1749-1751.
- Davis VE, Walsh MJ. Alcohol, amines and alkaloids: a possible biochemical basis for alcohol addiction. Science. 1970; 167: 1005-1007.
- Hamilton MG, Blum K, Hirst M. Identification of an isoquinoline alkaloid after chronic exposure to ethanol. Alcohol Clin Exp Res. 1978; 2: 133-137.
- Blum K. Alcohol & Opiates. New York, London: Academic Press. 1978.
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973; 179: 1011-1014.
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975; 88: 295-308.
- Blum K, Elston SF, DeLallo L, Briggs AH, Wallace JE. Ethanol acceptance as a function of genotype amounts of brain [Met] enkephalin. Proc Natl Acad Sci USA. 1983; 80: 6510-6512.
- Blum K, Briggs AH, Elston SF, DeLallo L, Sheridan PJ, Sar M. Reduced leucine-enkephalin--like immunoreactive substance in hamster basal ganglia after long-term ethanol exposure. Science. 1982; 216: 1425-1427.
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008; 28: 12672-12681.
- Blum K, Briggs AH, Elston SF, DeLallo L. Ethanol preference as a function of genotypic levels of whole brain enkephalin in mice. Toxicol Eur Res. 1981; 3: 261-262.
- Carenzi A, Biasini I, Frigeni V, Della Bella D. On the enzymatic degradation of enkephalins: pharmacological implications. Adv Biochem Psychopharmacol. 1980; 22: 237-246.
- Blum K, Briggs AH, Trachtenberg MC, Delallo L, Wallace JE. Enkephalinase inhibition: regulation of ethanol intake in genetically predisposed mice. Alcohol. 1987; 4: 449-456.
- Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, et al. Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct save. Alcohol. 1988; 5: 481-493.
- Brown RJ, Blum K, Trachtenberg MC. Neurodynamics of relapse prevention: a neuronutrient approach to outpatient DUI offenders. J Psychoactive Drugs. 1990; 22: 173-187.
- Blum K, Kozlowski G. Etanol and Neuromodulator interactions: A cascade model of reward In: Ollat H, Parvez S, Parvez H, editors. Alchol and Behavior. Prog in Alcohol Research. 1990; 131-150.
- Wise RA, Bozarth MA. Brain reward circuitry: four circuit elements "wired" in apparent series. Brain Res Bull. 1984; 12: 203-208.
- Blum K, Oscar-Berman M, Badgaiyan RD, Palomo T, Gold MS. Hypothesizing dopaminergic genetic antecedents in schizophrenia and substance seeking behavior. Med Hypotheses. 2014; 82: 606-614.
- Blum K, Payne J. Alcohol & The Addictive Brain. New York: The Free Press Simon & Schuster. 1990.
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990; 263: 2055-2060.
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991; 48: 648-654.
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996; 89: 396-400.
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995; 5: 121-141.
- Blum K, Wood RC, Braverman ER, Chen TJ, Sheridan PJ. The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes' theorem. Funct Neurol. 1995; 10: 37-44.
- Blum K, Simpatico T, Badgaiyan RD, Demetrovics Z, Fratantonio J, Agan G, et al. Coupling Neurogenetics (GARS) and a Nutrigenomic Based Dopaminergic Agonist to Treat Reward Deficiency Syndrome (RDS): Targeting Polymorphic Reward Genes for Carbohydrate Addiction Algorithms. J Reward Defic Syndr. 2015; 1: 75-80.
- Blum K, Gardner E, Oscar-Berman M, Gold M. "Liking" and "wanting" linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des. 2012; 18: 113-118.
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985; 9: 469-477.
- Beitscher-Campbell H, Blum K, Febo M, Madigan MA, Giordano J, Badgaiyan RD, et al. Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. J Behav Addict. 2016; 5: 533-534
- Merlo LJ, Sutton JA, Gold MS. Brief educational intervention to improve medical student competence in managing patients exposed to secondhand smoke. Substance abuse. 2014; 35: 163-167.
- Gold MS, Badgaiyan RD, Blum K. A Shared Molecular and Genetic Basis for Food and Drug Addiction: Overcoming Hypodopaminergic Trait/State by Incorporating Dopamine Agonistic Therapy in Psychiatry. Psychiatr Clin North Am. 2015; 38: 419-462.
- Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, Morse S, et al. Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms. J Addict Res Ther. 2012; 3: 139.
- Blum K, Chen TJ, Morse S, Giordano J, Chen AL, Thompson J, et al. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D(2) agonist therapy: part 2. Postgrad Med. 2010; 122: 214-226.
- Miller DK, Bowirrat A, Manka M, Miller M, Stokes S, Manka D, et al. Acute intravenous synaptamine complex variant KB220 "normalizes" neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports. Postgrad Med. 2010; 122: 188-213.
- Steinberg B, Blum K, McLaughlin T, Lubar J, Febo M, Braverman ER, et al. Low-Resolution Electromagnetic Tomography (LORETA) of changed Brain Function Provoked by Pro-Dopamine Regulator (KB220z) in one Adult ADHD case. Open J Clin Med Case Rep. 2016; 2: 1121.
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009; 3: 8-18.
- Li W, Li Q, Wang D, Xiao W, Liu K, Shi L, et al. Dysfunctional Default Mode Network in Methadone Treated Patients Who Have a Higher Heroin Relapse Risk. Sci Rep. 2015; 5: 15181.
- Lu H, Stein EA. Resting state functional connectivity: its physiological basis and application in neuropharmacology. Neuropharmacology. 2014; 84: 79-89.
- Lu H, Zou Q, Chefer S, Ross TJ, Vaupel DB, Guillem K, et al. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain connectivity. 2014; 4: 499-510.
- Moeller SJ, London ED, Northoff G. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity. Neuroscience and biobehavioral reviews. 2016; 61: 35-52.
- Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014; 51: 68-78.
- Sambataro F, Fazio L, Taurisano P, Gelao B, Porcelli A, Mancini M, et al. DRD2 genotype-based variation of default mode network activity and of its relationship with striatal DAT binding. Schizophrenia bulletin. 2013; 39: 206-216.
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015; 162: 712-725.
- Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, et al. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry. 2014; 19: 1037-1043.
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, et al. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res. 2014; 38: 2445-2453.
- Nestler EJ. Transgenerational Epigenetic Contributions to Stress Responses: Fact or Fiction? PLoS biology. 2016; 14.
- Blum K, Liu Y, Wang W, Wang Y, Zhang Y, Oscar-Berman M, et al. rsfMRI effects of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med. 2015; 127: 232-241.
- Ahn WY, Vasilev G, Lee SH, Busemeyer JR, Kruschke JK, Bechara A, et al. Decision-making in stimulant and opiate addicts in protracted abstinence: evidence from computational modeling with pure users. Front Psychol. 2014; 5: 849.
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl). 2005; 180: 612-623.
- Makhinson M, Gomez-Makhinson J. A successful treatment of buprenorphine withdrawal with the dopamine receptor agonist pramipexole. Am J Addict. 2014; 23: 475-477.
- SAMHSA. Medication-Assisted Treatment (MAT).
- Leon MD. Kids Are Dying Movie. In: Leon MD, editor. An American Epidemic Movie.
- Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008; 5: 24.
- Blum K, Gold M, Clark HW, Dushaj K, Badgaiyan RD. Should the United States Government Repeal Restrictions on Buprenorphine/Naloxone Treatment? Substance use & misuse. 2016; 1-6.
- Badgaiyan R, Sinha S, Blum K. Do We Really Need to Continue Pharmacotherapy for Opioid Use Disorder (OUD) Indefinitely. Reward Defic Syndr. 2015; 1: 16-19.
- Hill E, Han D, Dumouchel P, Dehak N, Quatieri T, Moehs C, et al. Long term Suboxone emotional reactivity as measured by automatic detection in speech. PLoS One. 2013; 8.
- Dackis CA, Gold MS, Sweeney DR, Byron JP Jr, Climko R. Single-dose bromocriptine reverses cocaine craving. Psychiatry research. 1987; 20: 261-264.
- Bogomolova EV, Rauschenbach IY, Adonyeva NV, Alekseev AA, Faddeeva NV, Gruntenko NE. Dopamine down-regulates activity of alkaline phosphatase in Drosophila: the role of D2-like receptors. J Insect Physiol. 2010; 56: 1155-1159.
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016; 68: 282-297.
- Blum K, Oscar-Berman M, Demetrovics Z, Barh D, Gold MS. Genetic Addiction Risk Score (GARS): molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS). Mol Neurobiol. 2014; 50: 765-796.
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, et al. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci USA. 2016; 113: 3024-3029.
- Borstein J. Malibu Beach Recovery Diet Cookbook. Malibu CA: Vidov Publishing Inc. 2015.
- Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC. Increased dopamine tone during meditation-induced change of consciousness. Brain Res Cogn Brain Res. 2002; 13: 255-259.
- Dahlgren A, Wargelius HL, Berglund KJ, Fahlke C, Blennow K, Zetterberg H, et al. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol and alcoholism. 2011; 46: 509-513.
- Blum K, Han D, Femino J, Smith DE, Saunders S, Simpatico T, et al. Systematic evaluation of "compliance" to prescribed treatment medications and "abstinence" from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014; 9.
- Blum K, Febo M, McLaughlin T, Cronje FJ, Han D, Gold SM. Hatching the behavioral addiction egg: Reward Deficiency Solution System (RDSS) as a function of dopaminergic neurogenetics and brain functional connectivity linking all addictions under a common rubric. J Behav Addict. 2014; 3: 149-156.
- McLaughlin T, Febo M, Badgaiyan R, D., Barh D, Dushaj K, Braverman ER., et al. KB220Z™ a Pro-Dopamine Regulator Associated with the Protracted, Alleviation of Terrifying Lucid Dreams. Can We Infer Neuroplasticity-induced Changes in the Reward Circuit? J Reward Defic Syndr Addict Sci. 2016; 2: 3-13.
