Research Article
Austin J Anal Pharm Chem. 2014;1(2): 1008.
Peroxidase Like Activity of Quinic Acid Stabilized Copper Oxide Nanosheets
Maddinedi SB and Mandal BK*
Department of Environmental and Analytical Chemistry Division, VIT University, India
*Corresponding author: :Mandal BK, Department of Environmental and Analytical Chemistry, School of Advanced Sciences, Vellore Institute of Technology (VIT) University, Vellore 632014, India.
Received: June 25, 2014; Accepted: July 16, 2014; Published: July 21, 2014
Abstract
Water-soluble copper oxide nanosheets (CuO nanosheets) were synthesized via a chemical reduction method and used as peroxidase enzyme mimetics. The synthesized CuO nanosheets were characterized by using XRD and TEM. The obtained CuO nanosheets in fact possessed an intrinsic enzyme like catalytic activity identical to that found in natural peroxidases, which have applications as detection tools and in water treatment.
Keywords: Water soluble CuO nanosheets; Peroxidase enzyme activity; Quinic acid
Introduction
Enzymes, a kind of proficient biocatalyst play key role in almost all in vivo reactions. Reactions catalyzed by enzymes are of extensive significance due to their high specificity, efficiency and need of mild reaction conditions. Additionally, enzyme based analysis has a broadscope of applications in various fields such as biochemistry, chemical technologies, clinical diagnosis and in environmental science. On the other hand, the availability of limited natural sources, instability at different temperatures, PH and high cost of purification has made enzymes restricted to limited applications. Hence there is a need for the construction of new stable, temperature and PH resistant, low cost materials which can replace the natural enzymes in their function and properties [1]. However, various enzyme mimetics have been synthesized for different enzymes such as serine protease [2], hydrolase [3], superoxide dismutase [4], dioxygenase [5,6], lipase [7], nitrile hydratases [8], phosphodiesterase [9-12], aldolase [13], cytochrome P450 [14], ligase [15] and acylase [16].
Now-a-days, much attention has been focused on the development of peroxidase mimetics due to its high importance in enzymatic analysis and in waste water treatment. Several peroxidase mimetics such as metal porphyrin [17], hemin [18], metal hexacyanoferrate [19,20], Schiff base complex [21], metal phthalocyanine [22], hemeatin [23] and carboxyl groups containing mesoporous polymers [24] have been used for enzymatic analysis.
Recently, scientists have proved that the iron oxide magnetic nanoparticles are found to possess intrinsic peroxidase mimetic activity [25]. Later, several nanoscale peroxidase mimetics have been developed to their potential applications [26–39]. The present study describes the intrinsic peroxidase activity of quinic acid stabilized copper oxide nanosheets sheets. Here, we have synthesized the water soluble copper oxide nanosheets and used as peroxidase enzyme mimics.
Chemicals and Materials
Experimental procedure
Copper (II) sulfate, Sodium hydroxide, Sodium borohydride, Quinic acid, Hydrogen peroxide, O-Dianisidine and all other solvents were obtained from Sigma-Aldrich Chemicals, Bangalore.
Preparation of CuO nanosheets
The copper oxide nanosheets were synthesized as per our reported procedure elsewhere [40]. Briefly, equal volume of aqueous NaOH (0.01 M) and NaBH4 (0.1 M) were added into a beaker containing the same amount of CuSO4 (0.01 M) and Quinic acid (0.02 M) mixture and stirred vigorously at room temperature (30°C). Reaction was continued for about a half an hour with stirring to obtain copper oxide nanosheets. The product was then purified by repeatedly washing with double distilled water and ethanol which was finally dried at 60°C in hot air oven.
Investigation of peroxidase like catalytic activity of CuO nanosheets
To examine the peroxidase like catalytic activity of the synthesized CuO nanosheets, the catalytic oxidation of o-dianisidine (as colourless peroxidase substrate, reduced form) was tested in the presence of H2O2 as oxidizing agent. The oxidation of o-dianisidine was carried out using phosphate buffer (pH 7.0) in the presence of CuO nanosheets (100 μl) for 200 seconds at 25°C. Later, hydrogen peroxide (10mM) was added to start the reaction. The catalytic activity of CuO nanosheets was carefully measured by the development of colour due to oxidation of o-dianisidine using UV-Visible spectrophotometer at 430 nm.
Kinetic parameters analysis
Steady-state kinetic experiments were done by changing the H2O2 concentration from 10 to 50mM at a fixed concentration of o-dianisidine (0.7mM) prepared in phosphate buffer (pH 7.0). The change in the absorbance was measured using a UV-Visiblespectrophotometer in the time scan mode at 430 nm. The catalytic parameters were then obtained by fitting the absorbance data to the Michaelis–Menten equation as,
V = Vmax[C]/Km + [C]
Where Vmax is the maximal velocity of reaction, V is the initial velocity and C represents the substrate concentration, and Km is the Michaelis-Menten constant.
Results and Discussion
Characterization of the copper oxide nanosheets
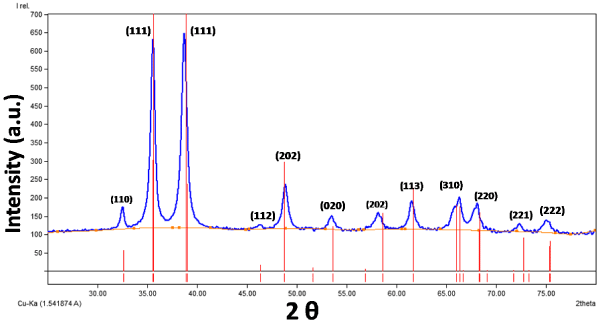
The copper oxide nanosheets were synthesized via a chemical reduction method. Figure 1 shows the XRD pattern of the produced CuO nanosheets which represents the formation of CuO nanosheets with a monoclinic structure (JCPDS No. 96-410-5686). Peaks in standard X-ray diffractogram of CuO obtained from JCPDS database had been matched with that of the obtained CuO nanosheets to find out any impurity present or not. Figure 1 shows the absence of characteristic impurity peaks in the XRD diffractogram of CuO nanosheets after comparison with standard diffractogram of CuO nanosheets which confirm the formation of pure CuO nanophase. The average crystalline size of the CuO nanosheets was calculated by using Debye-Scherrer formula as about 192 nm.
Figure 1: XRD pattern of the synthesized CuO nanosheets.
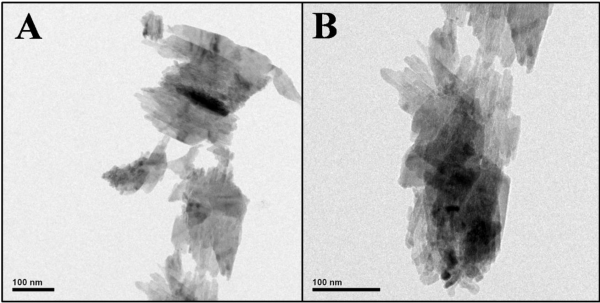
The morphology and size of the produced CuO nanosheets were analyzed by transmission electron microscopy (TEM). The TEM images (Figure 2) clearly show the presence of sheet like CuO nanostructures. However, the average size of the CuO nanosheets was found to be in good agreement with the XRD result.
Figure 2: HR-TEM images of the synthesized CuO nanosheets at 100 nm magnifications (A, B).
Peroxidase like activity of CuO nanosheets
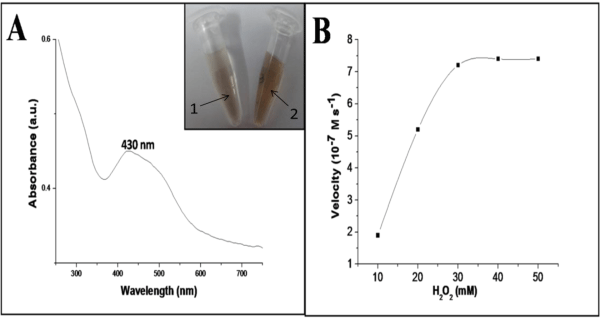
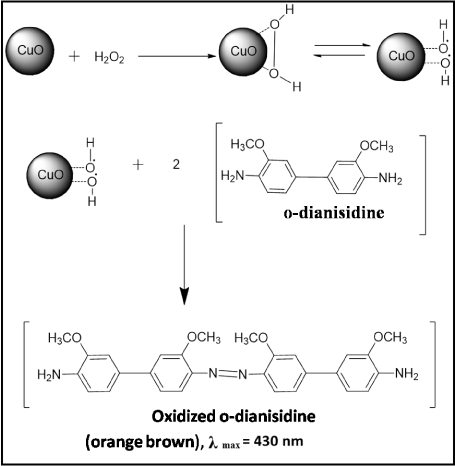
Figure 3A showed the typical UV-visible absorption spectrum of the reaction system containing CuO nanosheets, H2O2 and o-dianisidine shows an increase in the absorbance at 430 nm characteristic to the oxidized product of o-dianisidine, representing the typical peroxidase like activity of the CuO nanosheets. However, the reaction was studied in the absence of CuO nanosheets, with the addition of various concentrations of H2O2, but no significant color change was observed even at higher concentrations (> 50 mM) indicating inefficiency of H2O2 in oxidizing o-dianisidine. But in the presence of CuO nanosheets the orange brown color appeared which indicates the catalytic activity of CuO nanosheets when added to the o-dianisidine-H2O2 system (Figure 3A inset). The basic catalytic mechanism involves the formation of an intermediate complex by the two electron reduction of H2O2 to H2O in the presence of CuO nanosheets, and the o-dianisidine is bound to the formed intermediate complex by a nucleophilic attack, thus resulting the oxidation reaction to take place with a color change to orange brown (Figure 4).
Additionally, the enzymatic activity of the CuO nanosheets was further examined by taking steady state kinetics. The kinetic experimental information was obtained by changing the H2O2 concentration while keeping the constant o-dianisidine concentration. Figure 3B shows the typical Michaelis–Menten curves obtained for the CuO nanodispersion with H2O2 as substrates. Vmax and the Michaelis constant (Km) are recorded from the graph. The noticeable Km value of CuO nanosheets was compared with horseradish peroxidase (HRP) and other nano peroxidase mimetics, which confirmed that CuO nanosheets exhibits a considerable catalytic behavior to use as peroxidase mimetic (Table 1). The apparent Km and Vmax value of CuO nanosheets with H2O2 as substrate was found to be 15.8mM and 7.6 x10-7 M s-1, whereas that for HRP is 3.7 mM, 87 (10-7 M s-1, respectively under the same experimental conditions.
Figure 3: Typical UV-Visible spectra of o-dianisidine–H2O2–CuO nano reaction system (A) kinetic analysis of CuO nanodispersion with H2O2 as substrate (B); Inset: 1=without CuO nanosheets and 2= with CuO nanosheets.
Figure 4: Chemical reaction mechanism of CuO nanosheets relative to o-dianisidine-H2O2 system.
Catalyst
Km(mM)
Vmax / 10-8 M s-1
Reference
HRP
3.7
8.71
41
Co3O4
140.07
12.1
42
Fe3O4
154.0
9.78
41
Prussian blue Fe2O3
323.6
117
43
Palladium nanostructures
1064.0
443
44
ZnFe2O4
1.66
7.74
45
Platinum nanostructures
769
185
46
CuO nanosheets
15.8
0.77
Present work
Table 1: Comparison of the kinetic parameters of CuO nanosheets, HRP and other peroxidase mimics, where.
Conclusion
In conclusion, water-soluble copper oxide nanosheets were prepared and examined as peroxidase enzyme mimetics. The kinetic catalytic activity of the water-soluble CuO nanosheets is due to the high affinity of copper oxide sheets to hydrogen peroxide. Moreover, the excellent dispersibility of CuO nanosheets in the aqueous systems makes its applications easy in water systems. This work not only proves the intrinsic peroxidase activity of copper oxide nanosheets, but also leaves its scope of applications in medicine, environmental chemistry and biochemistry.
References
- Wiester MJ, Ulmann PA, Mirkin CA. Enzyme mimics based upon supramolecular coordination chemistry. Angew Chem Int Ed Engl. 2011; 50: 114-137.
- Mousumi G, Conroy JL, Christopher TS. Hydrolysis of Amides Catalyzed by 4-Hetrocyclohexanones: Small Molecule Mimics of Serine Proteases. Angew Chem Int Ed Engl. 1999; 38: 514-516.
- Ikeda H, Nishikawa S, Yamamoto Y, Ueno A. Homotropiccooperativity of cyclodextrin dimer as an artificial hydrolase. Journal of Molecular Catalysis A: Chemical. 2010; 328: 1-7.
- Cisnetti F, Lefevre AS, Guillot R, Lambert F, Blain G, Elodie AM, et al. A New Pentadentate Ligand Forms Both a Di- and a Mononuclear MnII Complex: Electrochemical, Spectroscopic and Superoxide Dismutase Activity Studies. Eur J Inorg Chem. 2007; 4472-4480.
- Chen K, Que Jr L. cis-Dihydroxylation of Olefins by a Non-Heme Iron Catalyst: A Functional Model for Rieske Dioxygenases. Angew Chem Int Ed Engl. 1999; 38: 2227-2229.
- Funabiki T, Yamazaki T, Fukui A, Tanaka T, Yoshida S. Oxygenative Cleavage of Chlorocatechols with Molecular Oxygen Catalyzed by Non-HemeIron (III) Complexes and Its Relevance to Chlorocatechol Dioxygenases. AngewChemInt Ed. 1998; 37: 513-515.
- Cacciapaglia R, Casnati A, Mandolini L, Reinhoudt DN, Salvio R, Sartori A, et al. Di- and Trinuclear Zn2+ Complexes of Calix[4]arene Based Ligands as Catalysts of Acyl and Phosphoryl Transfer Reactions. J Org Chem. 2005; 70: 624-630.
- Heinrich L, Aude MV, Li Y, Vaissermann J, Chottard JC. Cobalt (III) Complexes with Carboxamido-NandSulfenato-SorSulfinato-S Ligands Suggest that a Coordinated Sulfenate-Sis Essential for the Catalytic Activity of Nitrile Hydratases. Eur J Inorg Chem. 2001; 2203-2206.
- Molenveld P, Engbersen JF, Reinhoudt DN. Specific RNA Dinucleotide Cleavage by a Synthetic Calix Angew Chem Int Ed Engl. 1999; 38: 3189-3192.
- Manea F, Houillon FB, Pasquato L, Scrimin P. Nanozymes: gold-nanoparticle-based transphosphorylation catalysts. Angew Chem Int Ed Engl. 2004; 43: 6165-6169.
- Molenveld P, Engbersen JFJ, Reinhoudt DN. Dinuclearmetallo-phosphodiesterase models: application of calix[4]arenes as molecular scaffolds. Chem Soc Rev. 2000; 29: 75-86.
- Cacciapaglia R, Casnati A, Mandolini L, Peracchi A, Reinhoudt DN, Salvio R, et al. Efficient and selective cleavage of RNA oligonucleotides by calix[4]arene-based synthetic metallonucleases. J Am Chem Soc. 2007; 129: 12512-12520.
- Carboni D, Flavin K, Servant A, Gouverneur V, Resmini M. The first example of molecularly imprinted nanogels with aldolase type I activity. Chemistry. 2008; 14: 7059-7065.
- Heinrich V, Holzbecher M. A Bridged Porphyrinato(thiolato)iron (III) Complex as a Model of the Active Center of the Cytochrome P-450 Isozyme. Angew Chem Int Ed Engl. 1997; 36: 1442-1444.
- Han MJ, Yoo KS, Chang JY, Ha TK. 5-(beta-Cyclodextrinylamino)-5-Deoxy-alpha-D-Riboses as Models for Nuclease, Ligase, Phosphatase, and Phosphorylase. Angew Chem Int Ed Engl. 2000; 39: 347-349.
- Cacciapaglia R, Casnati A, Di Stefano S, Mandolini L, Paolemili D, Reinhoudt DN, et al. Dinuclear barium(II) complexes based on a calix[4]arene scaffold as catalysts of acyl transfer. Chemistry. 2004; 10: 4436-4442.
- Huang XM, Zhu M, Mao LY, Shen HX. Catalytic Determination of Hydrogen Peroxide by Using the Molybdenum-Porphyrin Complex as a Mimetic Enzyme of Peroxidase. Anal Sci. 1997; 13: 145-147.
- Fruk L, Niemeyer CM. Covalent hemin-DNA adducts for generating a novel class of artificial heme enzymes. Angew Chem Int Ed Engl. 2005; 44: 2603-2606.
- Li J, Qiu JD, Xu JJ, Chen HY, Xia XH. The Synergistic Effect of Prussian-Blue-Grafted Carbon Nanotube/ Poly (4-vinylpyridine) Composites for Amperometric Sensing. Adv Funct Mater. 2007; 17: 1574-1580.
- Chen W, Tang J, Cheng HJ, Xia XH. A simple method for fabrication of sole composition nickel hexacyanoferrate modified electrode and its application. Talanta. 2009; 80: 539-543.
- Tang B, Du M, Sun Y, Xu HL, Shen HX. The study and application of biomimic peroxidase ferric 2-hydroxy-1-naphthaldehyde thiosemicarbazone (Fe(III)-HNT). Talanta. 1998; 47: 361-366.
- Chen QY, Li CD, Zhu QZ, Zheng H, Xu JG. Application of iron tetrasulfonatophthalocyanine as a new mimetic peroxidase in the determination of hydrogen peroxide with p-hydroxyphenylpropionic acid as a substrate. Analytica Chimica Acta. 1999; 381: 175-182.
- Genfa Z, Dasgupta PK. Hematin as a peroxidase substitute in hydrogen peroxide determinations. Anal Chem. 1992; 64: 517-522.
- Liu S, Wang L, Zhai J, Luo Y, Sun X. Carboxyl functionalized mesoporous polymer: A novel peroxidase-like catalyst for H2O2 detection. Anal Methods. 2011; 3: 1475-1477.
- Gao L, Zhuang J, Nie L, Zhang J, Zhang YU, Gu N, ET AL. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology. 2007; 2: 577-583.
- Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008; 80: 2250-2254.
- Song Y, Wang X, Zhao C, Qu K, Ren J, Qu X, et al . Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chemistry. 2010; 16: 3617-3621.
- Zhang J, Zhuang J, Gao L, Zhang Y, Gu N, Feng J, et al. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere. 2008; 73: 1524-1528.
- Fan HM, Yi JB, Yang Y, Kho KW, Tan HR, Shen ZX, et al. Single-crystalline MFe(2)O(4) nanotubes/nanorings synthesized by thermal transformation process for biological applications. ACS Nano. 2009; 3: 2798-2808.
- Gao L, Wu J, Lylle S, Zehr K, Cao L, Gao D. Magnetite Nanoparticle-Linked Immunosorbent Assay. J Phys Chem C. 2008; 112: 17357-17361.
- Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010; 22: 2206-2210.
- Dai Z, Liu S, Bao J, Ju H. Nanostructured FeS as a mimic peroxidase for biocatalysis and biosensing. Chemistry. 2009; 15: 4321-4326.
- Ding N, Yan N, Ren C, Chen X. Colorimetric determination of melamine in dairy products by Fe(3)O(4) magnetic nanoparticles-H(2)O(2)-ABTS detection system. Anal Chem. 2010; 82: 5897-5899.
- He W, Liu Y, Yuan J, Yin JJ, Wu X, Hu X, et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials. 2011; 32: 1139-1147.
- Zuo X, Peng C, Huang Q, Song S, Wang L, Li D, et al. Design of a Carbon Nanotube/Magnetic Nanoparticle-Based Peroxidase-Like Nanocomplex and Its Application for Highly Efficient Catalytic Oxidation of Phenols. Nano Res. 2009; 2: 617-623.
- Cui R, Han Z, Zhu JJ. Helical carbon nanotubes: intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chemistry. 2011; 17: 9377-9384.
- Liu S, Tian J, Wang L, Luo Y, Chang G, Sun X. Iron-substituted SBA-15 microparticles: a peroxidase-like catalyst for H2O2 detection. Analyst. 2011; 136: 4894-4897.
- Liu S, Tian J, Wang L, Luo Y, Sun X. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Advances. 2012; 2: 411-413.
- Tian J, Liu S, Luo Y, Sun X. Fe(III)-based coordination polymer nanoparticles: peroxidase-like catalytic activity and their application to hydrogen peroxide and glucose detection. Catal Sci Technol. 2012; 2: 432-436.
- Maddinedi SB, Mandal BK, Pappu G, Anna KK, Ghosh AR. Synthesis of CuO nanosheets and its applications towards catalysis and antimicrobial activity. J. Indian Chem. Soc. [in press].
- de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol. 2012; 7: 821-824.
- Rodríguez-Lorenzo L, de la Rica R, Álvarez-Puebla RA, Liz-Marzán LM, Stevens MM. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat Mater. 2012; 11: 604-607.
- Rusling JF. Multiplexed electrochemical protein detection and translation to personalized cancer diagnostics. Anal Chem. 2013; 85: 5304-5310.
- Gao Z, Hou L, Xu M, Tang D. Enhanced Colorimetric Immunoassay Accompanying with Enzyme Cascade Amplification Strategy for Ultrasensitive Detection of Low-Abundance Protein. Scientific Reports. 2014; 3966.
- Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE. Point of care diagnostics: status and future. Anal Chem. 2012; 84: 487-515.
- Aragay G, Pino F, Merkoçi A. Nanomaterials for sensing and destroying pesticides. Chem Rev. 2012; 112: 5317-5338.