Review Article
Austin J Anal Pharm Chem. 2014;1(2): 1009.
Ionic Liquids as Solvents in Separation Processes
Jolanta Flieger1*, El Blicharska Grushka1 and Czajkowska-Zelazko A2
1Department of Analytical Chemistry, Medical University of Lublin, Poland
2Department of Haematological Diagnostics, Medical University of Lublin, Poland
*Corresponding author: :Jolanta Flieger, Department of Analytical Chemistry, Medical University of Lublin, 20-093 Lublin, Chodzki 4A, Poland.
Received: July 15, 2014; Accepted: July 21, 2014; Published: July 22, 2014
Abstract
The term ‘ionic liquids’ (IL), covers one of the broadest classes of chemical compounds of salt type of low melting temperature with appreciable wide liquid range. Within the last years, ILs has become mainstream solvents in different fields of chemistry. This has been possible owing to environmentally advantageous properties and possibility of adaptation of their structure to the specific task. Although at the beginning, ILs were mainly recognized as the reaction media in organic synthesis, an alternative to organic solvents, nowadays a growing interest of the analytical community with ILs can be observed. As a result, ILs has found a variety of applications in separation techniques. This overview presents chosen extraction techniques with particular emphasis on aqueous two-phase systems based on ionic liquids. The findings presented herein concern applications of ionic liquids as components of liquid/ liquid extraction systems, suitable for isolation of both organic compounds and inorganic ions.
Introduction
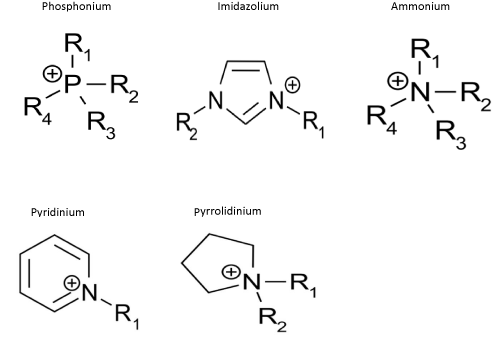
Ionic Liquids (ILs) are a new class of solvents that unlike other liquids have ionic structure. However, in contrast to classical salts, ILs is in the liquid state at temperatures below 100°C. Some of them, the so-called ‘room temperature ionic liquids’ (RTILs), melt already at room temperatures. There are also ionic liquids having a melting point below 0°C. Typical ionic liquid consists of organic cation (containing a phosphorus or nitrogen atom) and organic or inorganic anion. Due to the cation structure there are five basic classes of ionic liquids: ammonium, phosphonium, imidazolium, pyridinium, pyrrolidinium (Figure 1).
Figure 1: Common ILs cations.
Among organic or inorganic anions: halides, hexafluorophosphates, tetrafluoroborates, alkyl sulfates, tosylates, methanesulfonates and bis (trifluoromethylsulfonyl) imides have been distinguished.
The most characteristic features of ionic liquids are very low vapor pressure at room temperatures and thermal stability over a wide temperature range. ILs is generally non-flammable and is also very good solvents for both inorganic and organic compounds (from small, simple molecules to complex polymers). Owing to these properties, they are used as a new type of solvent with unique properties in such areas of chemistry as organic synthesis, electrochemistry, extraction, spectroscopy, mass spectrometry as well as separation techniques such as liquid and gas chromatography or capillary electrophoresis. Variety of applications of ionic liquids is also due to the possibility of designing their physicochemical properties by selecting appropriate cation and anion. For example, their solubility in water depends mainly on the anion, but may also be modified by the length of the cations alkyl substituent. Ionic liquids having in its composition such anions as halide, tetrafluoroborate, thiocyanate, sulfonic, trifluoroacetate and nitrate are soluble in water, while containing hexafluorophosphate or bis (trifluoromethylsulfonyl) imide anions they form two-phase systems with water. In turn, lengthening of the alkyl substituent in the cation of water-miscible ionic liquid increases the hydrophobicity and limits miscibility with water.
Number of applications on the use of ionic liquids in the sample pretreatment is constantly increasing [1-4]. Due to ecological nature, and the possibility of design, ionic liquids replace, more often than not, the organic solvents
Ionic Liquids in Liquid/Liquid Extraction
Ionic liquids have been applied for liquid-liquid extraction of metal cations, small organic molecules or large biomolecules as proteins [5]. As it can be observed, water-insoluble ionic liquids play a crucial role especially in the miniaturized version of liquid-liquid extraction technique [6] such as direct immersion, headspace, dynamic, hollow fiber protected liquid phase micro extraction (LPME), single drop micro extraction or dispersive liquid-liquid micro extraction and as modifiers of stationary phases in SPE.
In turn, water-soluble hydrophilic ionic liquids can also be used as extracting solvent, as they induce formation of aqueous biphasic systems (ABS) with suitable salting-out agents.
Ionic liquids in aqueous biphasic systems (ABS)
Aqueous biphasic systems were used already in the 80s of the previous century [7]. They were composed of polymers such as polyethylene glycols or dextrans [8]. After mixing solutions of two different polymers or a polymer and salt solutions at a suitable temperature, two water immiscible phases came to existence [9]. Decisive factor in the two phase formation is the enthalpy of interaction between the polymer molecules. If the value of the enthalpy is greater than the loss in entropy caused by phase separation, the ABS is formed. The analyte introduced to such a system is divided between the two phases. However most of the polymers used in the two-phase systems present a high viscosity and form a cloudy solution, which often makes further analysis difficult. Some polymers also cause interferences in the analysis, which greatly limits detection of the analyzed compounds and scope for the technique. To increase the application potential for ABS, polymers have been replaced by hydrophilic ionic liquids [10,11]. Abraham showed that this kind of ILs in combination with suitable kosmotropic agents form a new type of partitioning systems [12].
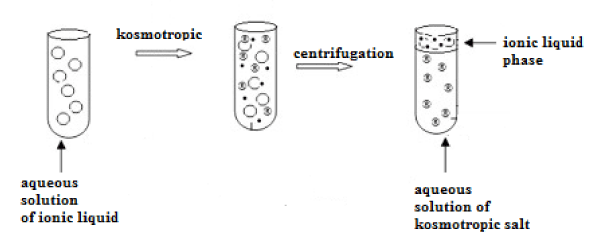
Such a two-phase system can be used for extraction as an alternative to conventional extracting systems of either liquid-liquid or liquid-solid type. The resulting extraction system is especially suitable for the analysis of aqueous samples. To create it, the right proportions of ionic liquid and kosmotropic salt should be found. Subsequent steps of ABS formation are presented in Figure 2.
Figure 2: Creation of aqueous bi-phase system (ABS) based on ionic liquid.
Mechanism of phase separation
Most of water-soluble ionic liquids are chaotropic agents, which mean that they perturb structure of hydrogen bonds network in water. Thus, they can be ‘salted out’ from aqueous solution by kosmotropic (water-structuring) salts. Combination of these two solutions with opposite nature causes solution to separate into two immiscible phases. The upper phase is usually an ionic liquid with lower water content, while the bottom one is water solution of the kosmotropic salt [13-16].
The mechanism of the ABS may be explained as follows: the ionic liquid competes with the salt ions for water molecules. Greater affinity of salt particles for water molecules causes their mutual attraction. This forces reduction of ILs is ions hydration and consequently decreases its water solubility. This leads to phase formation, consisting of ionic liquid and a small amount of water. This mechanism can also be explained on the basis of the thermodynamic theory of Gibbs ‘free hydration energy’. Kosmotropic ions having a large negative energy of hydration exhibit high affinity to water particles and attract them stronger than ionic liquids.
The salts were classified by the Hofmeister from kosmotropic (highly hydrated) to the chaotropic (weakly hydrated) according to their tendency to salting out of proteins [17]:
In aqueous biphasic systems the following salts are used: sodium, potassium or ammonium phosphates, the sulfates, carbonates and citrates. Alternatively, kosmotropic non-ionic agents as sugars [18-20] or amino acids [21,22] may be taken advantage of. Most common ionic liquids used in ABS are those composed of 1,3- dialkylimidazolium cation and a suitable anions that enable dissolving them in water: Cl-, Br-, I-, BF4-, MeSO4-, EtSO4-, NO3-. However, liquids containing phosphonium, pyrrolidinium or ammonium cations have also the potential to create aqueous two-phase systems. These ionic liquids were tested by Bridges as for their ability to create ABS in the presence of most kosmotropic salt - K3PO4 [14]. The possibility to create these systems is related to chaotropicity of cations and increases in the following order: P4444Cl > N4444Cl >> [C4py] Cl >> [C4mmim] Cl ≈ [C4mim] Cl. Louros et al. also examined influence of different cations on ability to form ABS [23]. They compared the imidazolium and phosphonium ionic liquids containing the same anions.
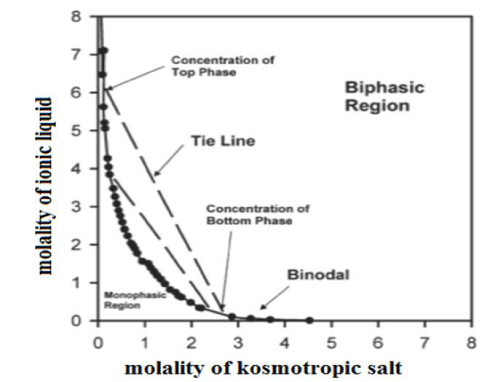
Tendency to formation of the two-phase systems can be illustrated by phase diagrams, which are described by an empirical model developed by Merchuk and co-workers [24]. Binodal represents equilibrium concentrations of the ionic liquid and kosmotropic salt at which a separation of two phases occurs. Binodal line is described by the best-fitting equation proposed by Merchuk:
Data for the graph can be determined experimentally by titration of the solution of a kosmotropic salt with the ionic liquid solution to the end, the so-called ‘cloud point’ which is the turbidity, indicating the formation of a separate phase.
Obtained binodal separates the curve space into two-phase regions situated above it and monophasic region located under the curve. A tight line connecting any two points of binodal determines quantitative composition of the two separated phases. Figure 3 shows the phase diagram drawn for exemplary ABS.
Figure 3: The phase diagram for exemplary ABS illustrating the binodal (-) and the tie lines (--).
Due to a very good solubility in water and relatively low cost, the most common ionic liquids used in aqueous biphasic systems are1,3- dialkylimidazolium chlorides, tetrafluoroborates and alkyl sulfates. Shehong and Li tested 1-butyl,3-methylimidazolium chloride in terms of salting out by various kosmotropic agents [11,25]. This ionic liquid can be salted out only by alkaline salts such as: KOH, NaOH, K3PO4, K2HPO4, K2CO3 or Na2HPO4. Salts with a lower pH such as KH2PO4, (NH4)2SO4, NaCl or KCl are not able to form a two-phase system with this ionic liquid. The ability to create two-phase system expresses the following series: K2CO3 ≈ K3PO4 ≈ K2HPO4>KOH. Tetrafluoroborates are able to form such system with more acidic or neutral salts [26]. The ability to create ABS with BMIMBF4is possible for the following series of salts: Na2CO3>Na2HPO4>Na2SO4>NaH2PO4>NaCl. Tetrafluoroborates 1-butyl, 3-methylimidazolium may also form two-phase systems when mixed with certain amino acids such as proline, L- serine, glycine, lysine [21,22]. Deive and co-workers tested a series of imidazolium alkyl sulfates (BMIM CnSO4, n=2,4,6,8) [27]. They proved that tendency to form two-phase systems increases with lengthening chain of the alkyl substituent in the anion. In this case salts can be used in order of highest capacity for salting out as follows: Na2CO3>K3PO4>K2CO3>(NH4)2SO4.
Without any doubt, the most famous ABS has been developed for butyl-methyl imidazolium chloride and K3PO4 as the salting-out agent. The equilibrium is achieved almost immediately after short centrifugation. Furthermore the upper phase of a two-phase systems containing aqueous concentrated solution of ionic liquid can be directly dispensed on the chromatographic columns, or after the dilution with an eluent. This simple technique of extraction has already found many practical applications being suitable for the extraction of both ionic and neutral compounds.
Application of ionic liquid aqueous biphasic system (ILABS) for extraction
Among the publications devoted to extraction with ABS, their large number takes into account a practical use. The analysis of complex biological materials such as human body fluids or environmental samples is a virtual analytical challenge. The isolation process usually proceeds across several stages, which may cause the analyte loss at each step. Reducing number of performed steps decreases the risk of analyte loss and shortens the time of analysis, which is of great importance for the analysis of less stable compounds. Taking into account the fact that the aqueous solutions of ionic liquids protect biomolecules from denaturation, ABS technique has proved to be highly competitive in relation to conventional organic solvents in the isolation of biomolecules. Aqueous biphasic systems were used for the extraction of proteins [28-31], hormones [32], alkaloids [33], vitamins [34], antibiotics [35-40], metal ions [41] from different biological samples such as fermentation broth, human urine, food and environmental aqueous samples.
Extraction of protein
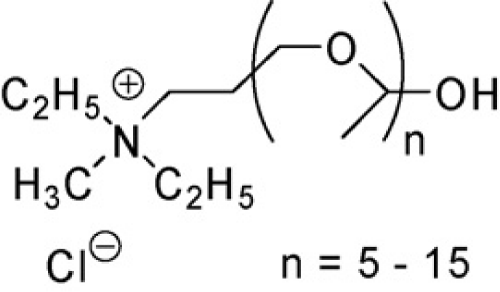
S. Dreyer et al using the ammonium ionic liquid Ammoeng 110 (Figure 4) has extracted such proteins as albumin, lysozyme, myoglobin, trypsin, and myoglobin [30]. As the salting-out agent phosphate buffer over a wide pH range was used. In the neutral solution trypsin, albumin and lysozyme were extracted in the range of 83-100%, while the myoglobin at the level of 60%. With the increase in pH overall negative charge of proteins increased as a result of surface functional groups ionization. Albumin showed 100% extraction in the whole pH range, while other proteins were strongly extracted only at higher pH values. In case of lysozyme and trypsin, total positive charge was reduced and charge of myoglobin turned into negative. Thus, selection of pH allows the selective extraction of albumin from a mixture of proteins. This also revealed that electrostatic forces are chiefly responsible for the mechanism of protein interactions with ionic liquids. The more negative charge on the protein surface, the stronger the interaction with the ionic liquid cation. Another factor considered affecting the change in extraction degree was the temperature in range of 4–70°C. The temperature increase during the formation of the two phase system, results in extraction decrease of the smaller molecular weight proteins.
Figure 4: Ammoeng 110.
Experiments published by Pei co-workers showed that apart from electrostatic interactions hydrophobic forces are equally responsible for protein extraction mechanism [28]. Proteins such as albumin, trypsin, cytochrome c and γ - globulins were extracted with 1,3-dialkylimidazolim bromide with different lengths of the cation substituent’s (C4,6,8 MIM Br). For salting out a phosphate buffer was used. In this case, the extraction of proteins was with 75-100% efficiency and reached maximum after application of the ionic liquid with the longest alkyl substituent in the cation. In the same time increase in temperature caused the increase in the degree of extraction. Also effect of the ionic liquid on the proteins activity was examined. It turned out that activity of trypsin in the IL solution decreases only slightly as compared to the ABS consisting of polymer and kosmotropic salt where there protein activity declines.
ABS extraction of protein (albumin and transferrin) from a real biological matrix like human urine was carried out [31]. The system used was composed of BMI MCl andK2HPO4. Recoveries of proteins on three different concentration levels of spiked human urine samples were in the range of 94-102%. The extraction was also highly selective, all concomitants like metal species remained in the K2HPO4 rich lower phase. According to the authors, the forces causing the extraction of proteins can be electrostatic interaction between negatively charged proteins and ionic liquid cations or only the salting out effect as a consequence of reduced protein solubility in a solution of K2HPO4.
Extraction of hormones, alkaloids and vitamins
He and co-workers determined testosterone and epitestosterone in human urine [32]. The extraction was carried out with 80-90 % efficiency. Analysis of urine samples was carried out in three stages, which consisted of enzymatic hydrolysis, protein removal with trichloroacetic acid, extraction with the use of 1-methyl-3- butylimidazolium chloride and K2HPO4. The ionic liquid upper phase was analyzed by high performance liquid chromatography. For both analytes the limit of detection reached 1ng/ml.
Aqueous phase systems based on ionic liquids were applied for isolation of alkaloids such as caffeine and nicotine from human urine [33]. The study showed that both compounds can be isolated in almost 100%by the use1,3 – dialkylimidazolium chloride. For salting out the most kosmotropic salt was utilized (K3PO4). The results showed that the extraction of alkaloids significantly improved samples in human urine in comparison to simpler aqueous phases. Authors concluded that the presence of a biological matrix containing additionally neutral salt NaCl and urea with chaotropic properties favored the alkaloid partitioning for the IL phase.
ABS systems were also used to quantify vitamin B12 in human urine [34]. After adequate preparation of urine samples (deproteinized and hydrolysis) vitamin B12 was extracted with 1-hexyl-3–methylimidazolium chloride that was further salted out by K2HPO4. The average extraction efficiency was 97 %.
Aqueous two phase system was applied for selective extraction of quinine from human plasma. Bi-phase was constructed from ionic liquid: butyl-methyl-imidazolium chloride following the addition of kosmotropic salts K3PO4 or KH23PO4. Quinine was determined in plasma samples after drinking of tonic containing quinine. Proposed strategy provides suitable sample purification and gives extraction yields in the range of 89-106 % [35].
Extraction of antibiotics
Isolation of antibiotics from fermentation broths followed by their separation and purification usually constitutes a large technological challenge. In order to test the activity, determine molecular structure and prepare the final form of drug, chemically pure compounds are required. Penicillin is one of the most popular and most commonly used antibiotics, thus streamlining and improving the efficiency of the production process as well as increasing its purity is essential.
Conducted in 2005 experiments by Luis how that the ABS composed of BMIM BF4 and NaH2PO4can be used for extraction of penicillin G from fermentation broth with more than 90 % efficiency [36]. Standard extraction with ethyl acetate is carried out in an environment of pH 2 while with the ionic liquid at pH ranging 4-5. As penicillin G is not stable in an environment with strongly acidic pH, the method presented by Luis allows maintaining the stability of the antibiotic particles and the same increases the efficiency of the extraction process.
Due to the widespread application of antibiotics both in human and veterinary therapy there is a risk of passing them into wastewater and next direct into the environment. Therefore the tests of drug levels are carried out in environmental samples. Han and co-workers used aqueous two-phase systems for extraction of antibiotics from water environmental samples (river, lake, medical sewage, pond water, ground water). They determined levels of macrolide antibiotics such asazithromycin and mydecamycin [37], roxithromycin [38], sulfadimidine [39], acetylspiramycin [40].
Optimizing the extraction systems for azithromycin, mydecamycin [37] and roxithromycin [38] binodal curves were done using 1-buty l,3-methylimidazolium tetrafluoroborate (BMIM BF4) and salts as Na2CO2, Na2HPO4, Na24SO4, NaH2PO4, NaCl and NaOH [37,38]. Next, partition coefficients for each antibiotic were determined as well as the effect of the added amount of salt in the extraction systems having the largest distribution coefficients. The highest degree of extraction of azithromycin was achieved with the system consisting of 10% BMIM BF4 and 24.5% Na2CO3 and of mydecamycin- 10% BMIM BF4 and 28.3% NaH2PO4. Under these conditions recovery of azithromycin and mydecamycin from environmental samples was from 91.8% to 96.2% and from 89.6% to 92.2%, respectively.
In turn, the system used for extraction of roxithromycin composed of BMIM BF4 and Na2CO3 had almost twice higher extraction capacity than traditionally used organic solvent [38]. Extraction of roxithromycin with ionic liquid exceeds 90%, whereas with an organic extractant reaches only about 50%.
ABS extraction procedure was also performed for analysis of sulfadimidine in river, lake and fish farm water [39]. In order to ensure proper pH of the extraction, (NH4) 2SO4 was selected as a salting-out agent. Ionic liquid capable of forming biphasic system with such salt (in a more acidic pH) was BMIM BF4. The maximum degree of extraction was achieved at pH between pK1 and pK2 of sulfadimidine, which suggests that the analyte in its neutral form interacts strongest way with the ionic liquid. In the extraction of real spiked water samples recovery between 101 and 107% were obtained.
ABS system was also used to analyze food samples such as milk, honey, drinking water [41]. With a system consisting of BMIM BF4 and sodium citrate single-step extraction of chloramphenicol was determined. In recovery tests of spiked samples with 10-100ng/mL of chloramphenicol, extraction efficiency ranged 90-102%. The method has also high selectivity, sugars and other substances present in food samples do not interfere in the assay.
Extraction of metal ions
Ionic liquids have been used for extraction of metals after addition of appropriate complexation reagent. Zhao distinguished three different procedures suitable for the extraction of metal cations from aqueous samples [42]. First method is based on Dai methodology applying crown ethers as complexing agent [43] second utilizes different anionic ligands for this purpose [44-48], and the last one proposes task-specific ILs (TSIL) with the covalent tethering of a cationic interchangeable functional group [49].
Imidazolium ionic liquids as solvents in liquid-liquid extraction systems have been most broadly described in literature, so far. The most promising examples of ionic liquids used for this purpose are 1-alkyl- 3-methylimidazolium hexafluorophosphate, tetrafluoroborate and bis (trifluoromethylsulphonyl) imide. Extraction of metal ions into ionic liquid phase requires addition of special ligand with the aim to enhance affinity of strongly hydrated ions to hydrophobic ILs phase. The most commonly used extractants are macrocyclic compounds like crown ethers, complexions (TBP-tributyl phosphate, CMPO-octyl (phenyl)-N,N-diisobutyl carbamoylmethyl phosphine oxide, PAN-(1-(2-pyridylazo)-2-naphtol, TAN-1-(2-thiazolyl)-2-naphtol, TODGA-N,N,N’,N’-tetra (octyl)diglycolamide) and other ligands like dithizone, 8-hydroxyquinoline, 1-(2-pyridylazo)-2-naphtol. Owing to above liquid-liquid extraction systems, significant improvement in extraction of alkali metals and lanthanides in comparison to traditional molecular organic solvent has been reported [50-56]. It has been also established that the distribution coefficients for metal cations were the most advantageous for shorter 1-alkyl-3-methyl imidazolium ILs. However it should be emphasized that lowering of ionic liquid hydrophobicity involves decreasing of the solubility of ligands in ionic liquid phase, finally worsening the extraction efficiency [56]. Another interesting observation has been made by Kozonoi et al. who reported better transferring of metal ions with greater charges to IL mona fluorobutyl sulphonate [57]. In turn, Domanska drew attention to importance of IL anion on extraction efficiency. It appeared that better extraction efficiency of Ag+ and Pb2+can be achieved with decreasing hydrophobicity of ILs anion [58].
A mechanism of metal ions extraction with imidazolium based ionic liquids can be described by cation-exchanging according to the following equation [59]:
Ligand addition is not “conditio sine qua non” for metal ions extraction by ionic liquids from aqueous media. Some metals like Zn2+ or Fe3+ can be directly transferred from aqueous phase into ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate [60], another one like Ce4+ from acidified water into 1-octyl-3-methylimidazolium hexafluorophosphate [61].
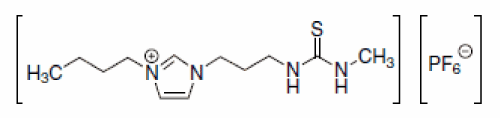
Furthermore, ionic liquid cations can be easily derivatized to include task-specific metal ligating groups. This causes significant enhancing of the partitioning of metal ions into the IL phase from water without necessity of additional ligands or extractants addition. Visser et al at the beginning of XXI century syntethsized imidazolium based ionic liquids derivatives that incorporated thiourea, thioether, urea [62] (Figure 5).
Figure 5: A thiourea derivatized ionic liquid.
These task-specific ionic liquid cations combining with the PF6- anion can act either as the hydrophobic insoluble in water solvent or metal ions such as Hg2+ and Cd2+ extractant. Functional, metal complexing disulphide, nitrile groups, can be also bonded to pyridinium, piperidinium or pyrrolidinium cation. Obtained in this way, ‘task-specific ionic liquids’ show affinity toward Hg2+, Cu2+, Pb2+, Ag+ [63].
Extraction systems containing ammonium or phosphonium ILs also extract quickly and almost completely metal ions without addition of any complexing agents. Methyltrioctylammonium chloride – [Aliquat 336] [Cl] seems to be the most efficient for binding Pd2+, Zn2+, Fe3+ [64,65]. In turn [Aliquat 336] [thiosalicylate] provides very strong binding abilities towards Cd2+ [66]. Fe3+ can be selectively separated from solution containing other metal ions by the use of methyltrioctylammonium salicylate [A336] [Sal]. Mechanism of this extraction was explained by Egorov [67] by the following equation:
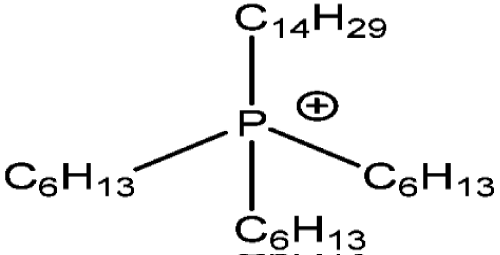
Recently phosphonium ionic liquids have been reported as useful solvents for metal ions extraction from aqueous media. Among various phosphonium ionic liquids, trihexyl (tetradecyl) phosphonium salts in mixture with toluene, propylene and especially butylene carbonate provided the highest extraction efficiency of such metal ions like Zn2+, Cd2+, Pd2+, Co2+ from chloride solutions [68-71] (Figure 6).
Figure 6: Trihexyl(tetradecyl)phosphonium IL cation [QP].
Metal ions are extracted in the form of chloro complexes according to the following reaction proposed by Kogelnig et al [72]:
Extraction efficiency increases with increasing hydrophilicity of IL anion, therefore the most advantageous value of recovery was observed for QP chlorides [QP] [Cl].
Aqueous bi-phase system (ABS) was also investigated for extraction of metal ions from wastewater samples [73].
ABS composed of ammonium ionic liquid – tetrabutylammonium bromide (TBA) and (NH4)2SO4 was utilized for Cr (VI) extraction. The mechanism responsible for the extraction process is the ion pair formation. The decisive factor was the pH. In acidic solution dominates form HCrO4 of Cr (VI) which forms a neutral ion pair with the ionic liquid cation. Such complex is more easily extracted into ionic liquids phase. The method is also selective; coexisting in the solution Cr (III) ions are not extracted. In the range of 0.05 to 40 mg Cr (VI) the recovery was over 97%.
Homogenous Liquid-Liquid Extraction (HLLE)
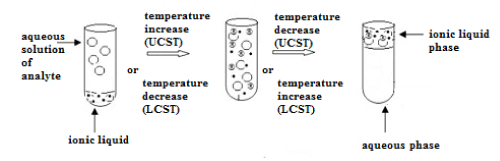
It is a common knowledge that liquid-liquid extraction is the basic technique used for the separation and purification. The distribution of a solute between two immiscible phases requires equilibration of this system accelerated by stirring or shaking. Application of immiscible with water, hydrophobic ionic liquids in such solvent extraction systems would have required even stronger agitation considering their higher viscosity in the aim to increase contact between both phases and finally increase the mass transfer between them. However, ionic liquids showing thermomorphic behavior, which are miscible with water above or below a certain temperature, do not require intensive mixing (Figure 7).
Figure 7: Thermomorphic behavior of ionic liquids with UCST and LCST.
By heating or cooling, a homogenous mixture can be obtained. Some ionic liquid solutions form one homogenous phase above the upper critical solution temperature (UCST) whereas other below a lower critical solution temperature (LCST). Such homogenous liquid-liquid extraction known also as phase-transition extraction or coalescence extraction is used for isolation of not only natural compounds [74,75] but also for metal ions [76-78]. Temperature induced phase separation can be obtained in systems containing for example 1-hexyl3-methylimidazolium tetrafluoroborate for isolation of silver, 4,4-bis-(dimethylamino)-thiobenzophenone, betainium bis (trifluoromethylsulfonyl)imide for isolation of rare earth metals. Another described HLLE system was applied for isolation of a larger selection of metals covering Ag, Cu, Zn, Fe, Co, Ni, In, Ga. Betainium bis (trifluoromethylsulfonyl) imide and betaine as the extracting agent appeared again to be suitable for efficient extraction. Homogenous phase was obtained after increasing the temperature above the UCST equals 55OC. Phase separation was achieved after cooling [79].
Conclusion
Although ILs has been already proved to present advantageous industrial applications, they still remain attractive for analytical chemists. Only laboratory scale can clarify rational rules for the best selection of ionic liquid for particular analytical task and practically important recycling procedures. From the practical viewpoint, we focused on utilising of ILs as solvents in extraction of different organic compounds and inorganic ions from biological and environmental samples. Liquid/liquid and aqueous two-phase systems based on ionic liquids appear to be very useful for that purpose. Without any doubt the application of ILs in these analytical approaches will enable researchers to achieve new, measurable benefits in the future.
References
- Flieger J, Czajkowska-Zelazko A. Ionic liquids in separation techniques: A Review: InTech. 2011; 311-343.
- Ren Q, Yang Q, Yan Y, Xing H, Bao Z, Su B, et al. Ionic-liquid-mediated liquid-liquid extraction: A Review. InTech. 2011; 343-361.
- Zaijun L, Xiulan S, Junkang L. Ionic liquid as novel solvent for extraction and separation in analytical chemistry: A Review: InTech. 2011; 153-181.
- Flieger J, Czajkowska-Zelazko A. Plasma sample preparation for solid phase extraction of chosen xenobiotics, utilizing salts differing in ion chaotropicity. Journal of Liquid Chromatography and Related Technology. 2014; 37.
- Huddleston JG, Willauer HD, Swatloski RP, Visser AE, Rogers RD. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chemical Communications. 1998; 1765-1766.
- Liu JF, Jiang GB, Chi YG, Cai YQ, Zhou QX, Hu JT, et al. Use of ionic liquids for liquid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Chem. 2003; 75: 5870-5876.
- Albertsson PA. Partition of Cell Particles and Macromolecules. Wiley, NowyJork. 1986.
- Walter H, Brooks DE, Fisher D. Partitioning in Aqueous Two-Phase System. Academic Press, NowyJork. 1985.
- Flory PJ. Principles of polymer chemistry. Cornell University Press, NowyJork. 1953.
- Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbreyand JD, et al. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc. 2003; 125: 6632-6633.
- Shehong L, Chiyang H, Huwei L, Kean L, Feng L. Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. Journal of Chromatography B Analyt Technol Biomed Life Sci. 2005; 826: 58-62.
- Abraham MH, Zissimos AM, Huddleston JC, Willauer HD, Rogers RD, Acree WE. Some novel liquid partitioning systems: Water-ionic liquids and aqueous biphasic systems. Industrial & Engineering Chemistry Research. 2003; 42: 413-418.
- Neves CM, Ventura SP, Freire MG, Marrucho IM, Coutinho JA. Evaluation of cation influence on the formation and extraction capability of ionic-liquid-based aqueous biphasic systems. J Phys Chem B. 2009; 113: 5194-5199.
- Bridges NJ, Gutowski KE, Rogers RD. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS). Green Chemistry. 2007; 9: 177-183.
- Pei Y, Wang J, Liu L, Wu K, Zhao Y. Liquid-Liquid Equilibrium Data for the Ionic Liquid N-Ethyl-Pyridinium Bromide with Several Sodium Salts and Potassium Salts. Journal of Chemical and Engineering Data. 2007; 52: 2026-2031.
- Deng Y, Chen J, Zhang D. Phase Diagram Data for Several Salt + Salt Aqueous Biphasic Systems at 298.15 K. Journal of Chemical & Engineering Data. 2007; 52: 1332-1335.
- Hofmeister F. ZurLehre von der Wirkung der Salze. Archiv Fur Experimentelle Pathologie Und Pharmacologie. 1888; 24: 247-260.
- Zhang Y, Zhang S, Chen Y, Zhang J. Aqueous biphasic systems composed of ionic liquid and fructose. Fluid Phase Equilibria. 2007; 257: 173-179.
- Wu B, Zhang Y, Wang H, Yang L. Temperature dependence of phase behavior for ternary systems composed of ionic liquid + sucrose + water. J Phys Chem B. 2008; 112: 13163-13165.
- Freire MG, Louros CLS, Rebelob LPN, Coutinho JAP. Aqueous biphasic systems composed of water -stable ionic liquid + carbohydrates and Their Applications. Green Chemistry. 2011; 13: 1536-1545.
- Domínguez-Pérez M, Tomé LIN, Freire MG, Marrucho IM, Cabeza O, Coutinho JAP. Extraction of biomolecules using aqueous biphasic systems formed by ionic liquids and aminoacids. Separation and Purification Technology. 2010; 72: 85-91.
- Zhang J, Zhang Y, Chen Y, Zhang S. Mutual Coexistence Curve Measurement of Aqueous Biphasic Systems Composed of [bmim][BF4] and Glycine, l-Serine, and l-Proline, Respectively. Journal of Chemical & Engineering Data. 2007, 52, 2488-2490.
- Louros CLS, Cléudio AFM, Neves CMSS, Freire MG, Marrucho IM, Pauly J, et al. Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. International Journal of Molecular Sciences. 2010; 11: 1777-1791.
- Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation. Studies on phase inversion. J Chromatogr B Biomed Sci Appl. 1998; 711: 285-293.
- Li S, He C, Liu H, Liand K, Liu F. Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. Journal of Chromatography B. 2005; 826: 58-62.
- Han J, Wang Y, Kang W, Li C, Yan Y, Pan J, et al. Phase equilibrium and macrolide antibiotics partitioning in real water samples using a two-phase system composed of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and an aqueous solution of an inorganic salt. Microchimica Acta. 2010; 169: 15-22.
- Deive FJ, Rodriguez A, Marrucho IM, Rebelo LPN. Aqueous biphasic systems involving alkylsulfate-based ionic liquids. The Journal of Chemical Thermodynamics. 2011; 43: 1565-1572.
- Pei Y, Wang J, Wu K, Xuan X, Lu X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Separation and Purification Technology. 2009; 64: 288-295.
- Du Z, Yu YL, Wang JH. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chemistry. 2007; 13: 2130-2137.
- Dreyer S, Salim P, Kragl U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochemical Engineering Journal.2009; 46: 176-185.
- Du Z, Yu YL, Wang JH. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chemistry. 2007; 13: 2130-2137.
- He C, Li S, Liu H, Li K, Liu F. Extraction of testosterone and epitestosterone in human urine using aqueous two-phase systems of ionic liquid and salt. J Chromatogr A. 2005; 1082: 143-149.
- Freire MG, Neves CMSS, Marrucho IM, Lopes JNC, Rebelob LPN, Coutinho JAP. High-performance extraction of alkaloids using aqueous two- phase systems with ionic liquids. Green Chemistry. 2010; 12: 1715-1718.
- Berton P, Monasterio RP, Wuilloud RG. Selective extraction and determination of vitamin B12 in urine by ionic liquid-based aqueous two-phase system prior to high-performance liquid chromatography. Talanta. 2012; 97: 521-526.
- Flieger J, Czajkowska-Zelazko A. Aqueous two phase system based on ionic liquid for isolation of quinine from human plasma sample. Food Chemistry. 2015; 166: 150-157.
- Lui Q, Xuesheng H, Wang Y, Yang P, Xia H, Yu J, et al. Extraction of penicillin G by aqueous two-phase system of [Bmim]BF4/NaH2PO4. Chinese Science Bulletin. 2005; 50: 1582-1585.
- Han J, Wang Y, Kang WB, Li CX, Yan XS, Pan JM, et al. Phase equilibrium and macrolide antibiotics partitioning in real water samples using a two-phase system composed of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and an aqueous solution of an inorganic salt. Microchimica Acta. 2010; 169: 15–22.
- Li CX, Han J, Wang Y, Yan YS, Xu XH, Pan JM, et al. Extraction and mechanism investigation of trace roxithromycin in real water samples by use of ionic liquid-salt aqueous two-phase system. Anal Chim Acta. 2009; 653: 178-183.
- Yu C, Han J, Wang Y, Yan Y, Hu S, Li Y, et al. Ionic liquid/Ammonium Sulfate Aqueous Two-phase System Coupled with HPLC Extraction of Sulfadimidine in Real Environmental Water Samples. Chromatographia. 2011; 74: 407–413.
- Wang Y, Han YA, Xie XQ, Li CX. Extraction of trace acetylspiramycin in real aqueous environments using aqueous two-phase system of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and phosphate. Central Europ J Chem. 2010; 8: 1185–1191.
- Han JA, Wang Y, Yu CL, Yan YS, Xie XQ. Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography. Analytical and Bioanalytical Chemistry. 2011; 399: 1295–1304.
- Zhao H, Xia S, Ma P. Use of ionic liquids as green solvents for extractions. Journal of Chemical Technology and Biotechnology. 2005; 80: 1089-1096.
- Dai S, Ju YH, Barnes CE. Solvent extraction of strontium nitrate by a crown ether using room temperature ionic liquids. Journal of the Chemical Society, Dalton Transactions. 1999; 1201-1202.
- Wei GT, Yang Z, Chen CJ. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. AnalyticaChimica Acta. 2003; 488: 183-192.
- Hirayama N, Deguchi M, Kawasumi H, Honjo T. Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta. 2005; 65: 255-260.
- Li Z, Wei Q, Yuan R, Zhou X, Liu H, Shan H, et al. new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta. 2007; 71: 68-72.
- Li Z, Lu N, Zhou X, Song Q. Extraction spectrophotometric determination of aluminium in dialysis concentrates with 3,5-ditertbutylsalicylfluorone and ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate. Journal of Pharmaceutical and Biomedical Analysis. 2007; 43: 1609-1614.
- Dadfarnia S, Shabani AM, Bidabadi MS, Jfari AA. A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in sample of nutritional interest. Journal of Hazardous Materials. 2010; 173: 534-538.
- Visser AE, Swatloski RP, Reichert WM, Mayton R, Sheff S, Wierzbicki A, et al. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: synthesis, characterization, and extraction studies. Environ Sci Technol. 2002; 36: 2523-2529.
- Dietz ML, Stepinski DC. A ternary mechanism for the facilitated transfer of metal ions into room temperature ionic liquids (RTILs): Implications for the “Greenness” of RTILs as extraction solvents. Green Chemistry. 2005; 7: 747-750.
- Dai S, Ju JH, Barnes CE. Solvent extraction of strontium nitrate by a crown ether using room temperature ionic liquid. Journal of the Chemical Society, Dalton Transactions. 1999: 1201-1202.
- Jensen MP, Dzielawa JA, Rickert P, Dietz ML. EXAFS investigations of the mechanism of facilitated ion transfer into a room-temperature ionic liquid. J Am Chem Soc. 2002; 124: 10664-10665.
- Nakashima K, Kubota F, Maruyama T, Goto M. Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Industrial and Engineering Chemistry Research. 2005; 44: 4368-4372.
- Nakashima K, Kubota F, Maruyama T, Goto M. Ionic liquids as a novel solvent for lanthanide extraction. Anal Sci. 2003; 19: 1097-1098.
- Stepinski DC, Jensen MP, Dzielawa JA, Dietz ML. Synergistic effects in the facilitated transfer of metal ions into room temperature ionic liquids. Green Chemistry. 2005; 7: 151-158.
- Luo H, Dai S, Bonnesen PV. Solvent extraction of sr2+ and cs+ based on room-temperature ionic liquids containing monoaza-substituted crown ethers. Anal Chem. 2004; 76: 2773-2779.
- Kozonoi N, Ikeda Y. Extraction mechanism of metal ion from aqueous solution to the hydrophobic ionic liquid, 1-butyl-3-methylimidazolium nonafluorobutanesulfonate. Monatshefte fur Chemie. 2007; 138: 1145-1151.
- Domanska U, Rekawek A. Extraction of metal ions from aqueous solutions using imidazolium based ionic liquids. Journal of Solution Chemistry. 2009; 38: 739-751.
- Regel-Rosocka M, Wisniewski M. Ionic liquids in separation of metal ions from aqueous solutions: A Review. InTech. 2011; 375-399.
- Perez de los Rios A, Hernandez-Fernandez FJ, Sanchez-Segado S, Lozano LJ, Moreno JI, Godinez C. Selective separation of Zn(II) and Fe(III) from acidic media using ionic liquids as sole extraction agents. Chemical Engineering Transactions. 2010; 21: 625-630.
- Zuo Y, Liu Y, Chen J, Li DQ. The separation of cerium (IV) from nitric acid solutions containing thorium (IV) and lanthanides (III) using pure [C8mim]PF6 as extracting phase. Industrial and Engineering Chemistry Research. 2008; 47: 2349-2355.
- Visser AE, Swatloski RP, Reichert WM, Mayton R, Sheff S, Wierzbicki A, et al. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: synthesis, characterization, and extraction studies. Environ Sci Technol. 2002; 36: 2523-2529.
- Papaiconomou N, Lee JM, Salminen J, von Stosch M, Prausnitz JM. Selective extraction of copper, mercury, silver and palladium ions from water using hydrophobic ionic liquids. Industrial and Engineering Chemistry Research. 2008; 47: 5080-5086.
- Giridhar P, Venkatesan KA, Srinivasan TG, Vasudewa Rao PR. Extraction of fission palladium by Aliquat 336 and electrochemical studies on direct recovery from ionic liquid phase. Hydrometallurgy. 2006; 81: 30-39.
- Perez de los Rios A, Hernandez-Fernandez FJ, Sanchez-Segado S, Lozano LJ, Moreno JI, Godinez C. Selective separation of Zn(II) and Fe(III) from acidic media using ionic liquids as sole extraction agents. Chemical Engineering Transactions. 2010; 21: 625-630.
- Kogelnig D, Stojanovic A, Galanski M, Groessl M, Jirsa F, Krachler R, et al. Greener synthesis of new ammonium ionic liquids and their potential as extracting agents. Tetrahedron Letters. 2008; 49: 2782-2785.
- Egorov VM, Djigailo DI, Momotenko DS, Chernyshov DV, Torocheshnikova II, Smirnova SV, et al. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent for transition metal ions. Talanta. 2010; 80: 1177-1182.
- Marszalkowska B, Regel-Rosocka M, Nowak L, Wisniewski M. Quaternary phosphonium salts as effective extractants of Zinc (II) and Iron (III) ions from acidic pickling solutions. Polish Journal of Chemical Technology. 2010; 12: 1-5.
- Nowak L, Regel-Rosocka M, Marszalkowska B, Wisniewski M. Removal of Zn (II) from chloride acidic solutions with hydrophobic quaternary salts. Polish Journal of Chemical Technology. 2010; 12: 24-28.
- Regel-Rosocka M. Extractive removal of zinc (II) from chloride liquors with phosphonium ionic liquids/toluene mixtures as novel extractants. Separation and Purification Technology. 2009; 66: 19-24.
- Regel-Rosocka M. A review on methods of regeneration of spent pickling solutions from steel processing. J Hazard Mater. 2010; 177: 57-69.
- Kogelnig D, Stojanovic A, Jirsa F, Körner W, Krachler R, Keppler BK. Transport and separation of iron (III) from nickel (II) with the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Separation and Purification Technology. 2010; 72: 56-60.
- Akama Y, Sali A. Extraction mechanism of Cr (VI) on the aqueous two-phase system of tetrabutylammonium bromide and (NH(4))(2)SO(4) mixture. Talanta. 2002; 57: 681-686.
- Lamb JD, Peterson RT. Coalescence extraction-A novel, rapid means of performing solvent extractions. Separation Science and Technology. 1995; 30: 3237-3244.
- Schaadt A, Bart HJ. Coalescence extraction-A benign extraction tool. Chemical Engineering and Technology. 2003; 26: 469-472.
- Hosseini MH, Alizadeh N. Coalescence extraction system for rapid efficient and selective separation of zirconium and hafnium. Industrial and Engineering Chemistry Research. 2010; 49: 7068-7073.
- Alizadeh N, Ashtari K. Coalescence extraction of silver (I) using temperature – induced phase separation (TIPS) process. Separation and Purification Technology. 2005; 44: 79-84.
- Vander Hoogerstraete T, Onghena B, Binnemans K. Homogeneous liquid-liquid extraction of rare earths with the betaine-betainium bis(trifluoromethylsulfonyl)imide ionic liquid system. Int J Mol Sci. 2013; 14: 21353-21377.
- Hoogerstraete TV, Onghena B, Binnemans K. Homogenous liquid-liquid extraction of metal ions with a functionalized ionic liquid. The Journal of Physical Chemistry Letters. 2013; 4: 1659-1663.