Editorial
Austin J Anal Pharm Chem. 2014;1(5): 1025.
Carbon Nanotube Chemically Modified Electrode as a Biomimic Cellular System for the Assessment of Phenolic Pharmaceutical Drugs and Cell Interactions: A New Proposal
Annamalai Senthil Kumar*
Environmental and Analytical Chemistry, Division, School of Advanced Sciences, Vellore Institute of Technology, Tamil Nadu
*Corresponding author: :Annamalai Senthil Kumar, Environmental and Analytical Chemistry, Division, School of Advanced Sciences, Vellore Institute of Technology, Tamil Nadu.
Received: November 14, 2014; Accepted: November 15, 2014; Published: November 17, 2014
Editorial
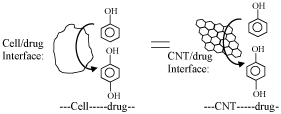
Understanding of the precise pharmacokinetics of drugs is a challenging interest. Although well sophisticated analytical instrumentation techniques such as high-performance liquid chromatography and spectroscopes are available, exact detail about the interaction and chemical species/intermediates formed on the drug/cell interface can be predicted easily. Infact it is highly difficult to predict the intermediate species formed on the pharmacokinetics. Here in, I propose, carbon nanotube (CNT), as a modified electrode, as a biomimetic cellular system to study the in-situ interaction between the phenolic pharmaceutical drugs and cell. Note that both "cell" and "CNT" having several similarities such as nanodimention, composed of carbon (in cell carbon is a major content) and allowing to adsorb organic and pharmaceutical compounds on the surface (Scheme 1). Since the CNT has well-ordered structure and conducting in nature the electron-transfer step happening on the CNT/drug interface can be easily probed in-sitully by simple voltammetric technique like cyclic voltammetry and pulse voltammetry. As a preliminary study, we have examined the interaction of phenol, tetracycline, amoxicillin, penicillin, and ampicillin on a CNT modified glassy carbon electrodes, designated as GCE/CNT, by cyclic voltammetric technique in a near physiological pH, pH 7 phosphate buffer solution [1-3]. It is interesting to notice that phenol, tetracycline and amoxicillin, which are having a mono-phenolic group, get easily oxidized either at +500 mV vs Ag/AgCl (case-1) or -450 mV vs Ag/AgCl (case-II) in pH 7 PBS to corresponding p-hydroxy benzene derivatives (Scheme 1) and it is showing a well-defined surface-confined redox feature at ~0 V vs Ag/AgCl selectively. Control experiments with non-phenolic drugs such as penicillin and ampicillin failed to show any such conversion. In the case-I mechanism, the phenolic group gets oxidized directly as a phenolic radical and subsequently to hydroquinone derivative. While in the case-II mechanism, the hydrogen peroxide, which was formed at negative potential, -450 mV vs Ag/AgCl in pH 7, as intermediate species interacted with the phenolic drugs and oxidized those molecules as p-hydroxy benzene derivatives. Some of the results obtained in the electrochemical system can be correlatable to pharmokinetics of the drugs. Detailed investigation using some model/s to understand the pharmacokinetics both on CNT and cellular system is necessary to further conclude the proposal.