
Review Article
Austin J Anal Pharm Chem. 2015;2(1): 1032.
Asymmetric Sulfenylation of Glycine Derivative Catalyzed by the Novel Chiral Phase Transfer Catalysts
Xiaoyang Dong, Pei Xu , Zhiyu Wang, Daorui Huang and Dai Zhenya*
Department of Pharmaceutical Chemistry, School of Pharmacy, China Pharmaceutical University, China
*Corresponding author: Dai Zhenya, Department of Pharmaceutical Chemistry, School of Pharmacy, China Pharmaceutical University, China.
Received: January 25, 2015;Accepted: February 12, 2015; Published: February 13, 2015;
Abstract
A new type of chiral phase transfer catalysts were synthesized and applied in the asymmetric sulfenylation of glycine derivative with moderate to high yields and moderate to high ees (58-88%).
Keywords: Chiral phase transfer catalysts; Asymmetric sulfenylation; Glycine derivatives
Introduction
Chiral unnatural sulfenylated amino acids play an important role in pharmaceutical industry and the synthesis of them are of great importance. The asymmetric sulfenylation of glycine derivatives with chiral phase transfer catalysts is one of the most important methods to prepare the chiral unnatural sulfenylated amino acids [1]. In recent years, chiral phase transfer catalysts derived from cinchona alkaloids have been a hotspot in asymmetric catalysis. Up to date, such kind catalysts can be divided into three generations [2-5]. As is shown in Figure 1: the first generation: R=H, Ar=Phenyl; the second generation: R=Allyl, Ar=Phenyl; and the third generation: R=Alkyl, Ar=Anthracyl. Deng et al. reported that the second generation of the catalysts could catalyze the asymmetric Darzens reaction with high yield and excellent enantioselectivity [6], Waser et al. reviewed the catalyzed asymmetric reactions catalyzed by the bi functional ammonium catalysts [7], Maruoka et al. reviewed the asymmetric phase transfer catalysis with chiral ammonium catalysts derived from cinchona and chiral C2-type ammonium catalysts [8].
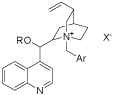
Figure 1: Three generations of the chiral phase transfer catalysts derived
from cinchona.
According to E. J. Corey’s theory about the chiral phase transfer catalysts derived from cinchona alkaloids, if the bridgehead nitrogen of a cinchona alkaloid quaternary salt is taken to be at the center of a tetrahedron, the phase transfer catalyst should be structured so as to provide steric effect which prevents close approach of the counter-ion to three of the faces of this tetrahedron, while the fourth face should be sufficiently open to allow close contact between the substrate counter-ion and N+ ( Figure 2).
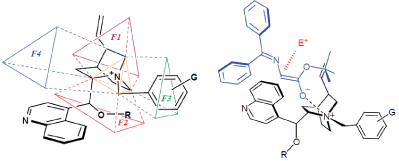
Figure 2: E. J. Corey’s model about the chiral phase transfer catalysts.
The natural chiral carbon atoms of cinchona alkaloids are essential for their enantioselectivity. The hydroxyl group and bridgehead nitrogen of cinchona alkaloids parent nucleus are two key groups, and the modification of them would directly affect the enantioselectivity based on Corey’s theory. And till now all modification were on the two groups respectively, only few papers [9] reported the modification on both two groups with one single reagent at the same time. We tried to realize the modification on the two groups at the same time, the two groups could be combined together to form a new cycle. Such structure would be more stable to shield the three faces of the tetrahedron, and the stereo selectivity would be enhanced. Here in we designed a new series of chiral phase transfer catalysts (1, 2, 3, 4) based on the imagination. Those catalysts all had a six-member rigid ring structure, which would be more stable and have the better enantioselectivity. We expected that the enantioselectivity would be increased by the formation of the rigid ring of those catalysts (Figure 3).
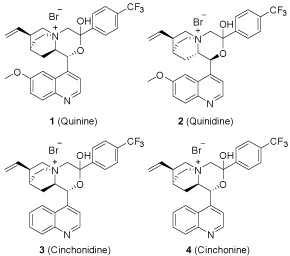
Figure 3: Catalyst 1-4.
A new series of chiral phase transfer catalysts were derived from cinchona alkaloids with 2-bromo-1-(4-(trifluoromethyl) phenyl) ethan-1-one (5). First, Chem-3D was used to simulate Three- Dimensional of the designed catalysts in order to determine the feasibility of this kind of compounds and their minimum energy state. All the four isomers of cinchona alkaloids could form the purposed conformation of six-member ring structure, and the energy was close to each other and was near 65 kcal/mol. In accordance to the reference 9, only one anomer was formed in the condensation of the a-halogen ketone with cinchona alkaloids. Then the catalysts were synthesized, the 2-bromo-1-(4-(trifluoromethyl)phenyl)ethan-1-one (5) was prepared by 1-(4-(trifluoromethyl)phenyl)-ethan-1-one and bromine [10], then the four isomers were stirred with 5 in THF for 8 hours (Figure 4), and 1, 2, and 3 could be achieved in 97-99% yields and no product of 4 was achieved.
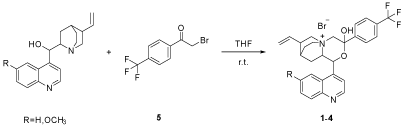
Figure 4: Synthesis of catalyst 1-4.
Then all the three catalysts were applied in the asymmetric sulfenylation of glycine derivative to evaluate their catalytic efficiency and enantioselectivity. The asymmetric benzylation of glycine derivative (6) was chosen as a model reaction to optimize the reaction condition and the results were list in Table 1.
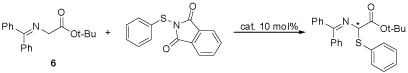
Figure 5: The asymmetric catalytic sulfenylation of glycine derivatives with
different catalysts under different reaction conditions.
Entry
Catalyst
Temperature
Base
Solvent
ee(%)c
1
1
10°C
50%KOH
Toluene
67
67
2
2
10°C
50%KOH
Toluene
64
44
3
3
10°C
50%KOH
Toluene
65
84
4
3
4°C
50%KOH
Toluene
60
84
5
3
20°C
50%KOH
Toluene
73
76
6
3
10°C
50%NaOH
Toluene
60
78
7
3
10°C
CsOH.H2O
Toluene
68
82
8
3
10°C
50%KOH
CH2Cl2
74
55
a. The reaction was carried out with 1.1 equiv. of sulfenylation reagent and 20.0 equiv.of 50% alkaline solution in the presence of 10 mol% 1-3 in different organic agents under the given conditions.
b. Isolated yields.
c. Enantiopurity was determined by HPLC analysis of benzylated imine using a chiral colume (DAICEl Chiralcel OD-H) with hexanes/ i-PrOH (volume ratio = 99.5:0.5) as a solvent.
Table 1: The asymmetric catalytic sulfenylation of glycine derivatives with different catalysts under different reaction conditions.
As shown in Table 1, of all the catalysts investigated, catalyst 3 was the best one with the highest yield and best ee value. The enantioselectivities did not change much as temperature changed, and 10°C was the best temperature. Different bases were also investigated, and 50% of the aqueous potassium was better than aqueous sodium hydroxide and solid cesium hydroxide. Of all the solvents investigated, toluene gave the best enantioselectivity. So the optimized reaction condition was with 3 as catalyst, with 50% aqueous of KOH as base, with toluene as the solvent and at 10°C. Having found the optimized reaction conditions, we tried to investigate the other sulfenylation reagents and the results were listed in Table 2.
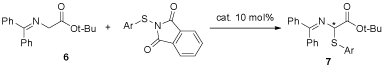
Figure 6: Catalytic asymmetric sulfenylation of glycine derivatives with catalyst 3a
Entry
Product
Ar
Times(h)
Yield(%)b
ee(%)c
1
7a
Ph
18
65
84
2
7b
4-methylpheny
48
55
70
3
7c
4-methoxyphenyl
36
54
88
4
7d
4-chlorophenyl
24
85
50
5
7e
4-bromophenyl
18
80
45
6
7f
benzyl
18
60
85
- Reaction was carried out with 1.1 equiv. of sulfenylation reagents and 20.0 equiv. of 50% aqueous KOH in the presence of 10 mol% 3 in toluene under the given conditions.
- Isolated yields.
- Enantiopurity was determined by HPLC analysis of benzylated imine using a chiral colume (DAICEl Chiralcel OD-H) with hexanes/ i-PrOH (volume ratio = 99.5:0.5) as a solvent.
Table 2: Catalytic asymmetric sulfenylation of glycine derivatives with catalyst 3a
As was shown in Table 2, the better yields while the worse enantioselectivities were achieved for the sulfenylation reagents with electron-withdrawing group on the aromatic ring, while the lower yields and the better enantioselectivities were achieved for the sulfenylation reagents with electron-donating group on the aromatic ring, for the sulfenylation reagent with methoxy group on the aromatic ring, the best enantioselectivity was achieved as 88%.
In conclusion, we designed and synthesized a series of new chiral phase transfer catalysts (1-3) derived from cinchona alkaloids and their applications in the asymmetric sulfenylation of glycine derivative were also investigated, and mediate to high yields and mediate to high ees were achieved with 3 as catalyst. Further work is under to under the mechanism of the reaction and to increase the enantioselectivity.
Acknowledgment
We were thankful to the National Natural Science Foundation of China for their financial support (No. 21102180).
References
- Martin L, Cornille F, Turcaud S, Meudal H, Roques BP, Fournié-Zaluski MC. Metallopeptidase inhibitors of tetanus toxin: A combinatorial approach. J Med Chem. 1999; 42: 515-525.
- Dolling UH, Davis P, Grabowski EJJ. Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis. J. Am. Chem. Soc, 1984, 106, 446-447.
- O'Donnell MJ, Wu S, Huffman JC. A new active catalyst species for enantioselective alkylation by phase-transfer catalysis. Tetrahedron, 1994, 50, 4507-4518.
- Corey EJ, Xu F, Noe MC. A rational approach to catalytic enantioselective enolate alkylation using a structurally rigidified and defined chiral quaternary ammonium salt under phase transfer conditions. J. Am. Chem. Soc., 1997, 119, 12414-12415.
- Lygo B, Wainwright PG. A new class of asymmetric phase-transfer catalysts derived from Cinchona Alkaloids-application in the enantioselective synthesis of a-amino acids. Tetrahedron Lett, 1997, 38, 8595-8598.
- Liu Y, Provencher BA, Bartleson KJ, Deng L. Highly enantioselective asymmetric Darzens reactions with a phase transfer catalyst. Chem. Sci 2011, 2, 1301-1304.
- Novacek J, Waser M. Bifunctional quaternary ammonium salt catalysts: a rapidly emerging class of powerful asymmetric catalysts. Eur. J. Org. Chem. 2013, 4, 637-648.
- Hashimoto T, Maruoka K. Recent development and application of chiral phase transfer catalysts. Chem. Rev. 2007, 107, 5656-5682.
- Xiong RG, Song YM, Ye Q. Quarternary ammonium salt of quinine compound, its preparation method and use. CN 1765503.
- Fan Z, Shi Z, Zhang H, Liu X, Bao L, Ma L, et al. Synthesis and biological activity evaluation of 1, 2, 3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J. Agr. Food Chem., 2009, 57, 4279-4282.