
Research Article
Austin J Anal Pharm Chem. 2015; 2(6): 1057.
Design of Experiment based Optimized RP-HPLC Method for Simultaneous Estimation of Amlodipine and Valsartan in Bulk and Tablet Formulations
Mittal A¹*, Imam SS², Parmar S³, Gilani SJ² and Taleuzzaman M²
¹Department of Pharmaceutics, KIET School of Pharmacy, Ghaziabad 201206, Uttar Pradesh, India
²Glocal School of Pharmacy, Glocal University, Saharanpur-247121, Uttar Pradesh, India
³H.R. Institute of Pharmacy, Ghaziabad-201206, Uttar Pradesh, India
*Corresponding author: Ashu Mittal, Department of Pharmaceutics, KIET School of Pharmacy, UP, India.
Received: November 27, 2015; Accepted: December 29, 2015;Published: December 31, 2015
Abstract
A simple and precise RP-HPLC method was developed and validated for the simultaneous determination of amlodipine and valsartan combination in bulk and tablet dosage form. This method involves the design of experiments approach for the optimization of mobile phase by taking methanol, pH and flow rate as the dependent variable and their effect was seen on retention time of amlodipine (4.35min) and valsartan (10.26 min). A linear response was observed over the concentration range of 5–50 μg/mL for amlodipine and 10-100μg/ mL for valsartan. Limit of detection (LOD) and limit of quantitation (LOQ) for amlodipine were found to be 1.20μg/mL and 3.71μg/mL, and for valsartan were 1.45μg/mL and 4.39μg/mL, respectively. The method was successfully validated in accordance with ICH guideline acceptance criteria for linearity, accuracy, precision, specificity, robustness. The analysis concluded that the method was selected for simultaneous estimation of amlodipine and valsartan, further can be potentially used for estimation of these drugs in combined dosage form.
Keywords: Box Behnken Design; Amlodipine; Optimization; Valsartan
Introduction
Liquid chromatography is the most widely used analytical tools in the pharmaceutical industry and reversed-phase is the most frequently used mode. During the drug development process, liquid chromatography methods are used to determine the purity of the drug substance (active pharmaceutical ingredient) and drug product. There are a limited number of HPLC methods are available for regular routine analysis of AML and VAL in combination pharmaceutical formulations. The use of high cost solvents is found to be highly complex and are associated with increasing numbers of process variables, which makes them less acceptable for routine analysis. On the other hand, HPLC methods require strong optimization of process variables such as mobile phase composition, pH of the mobile phase and flow rate.
Amlodipine (AML) (Figure 1) is a potent calcium channel blocker and belongs to the dihydropyridine class of calcium channel blockers (CCBs) and most widely used class of CCBs [1]. Amlodipine with its intrinsically long half-life alone or together with β-blocker, is likely to produce superior ischaemia reduction in clinical practice when patients frequently forget to take medication or take doses irregularly [2,3]. The literature survey revealed HPLC [4-6], RP-HPLC [7-10], LC-MS [11-12] for are reported for simultaneous estimation of AML alone or in combination with other anti-hypertensive agents.
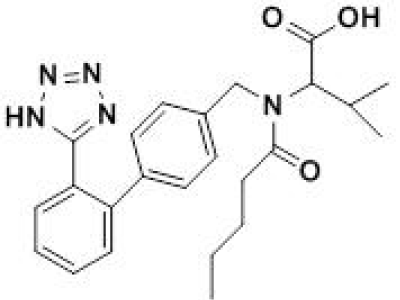
Figure 1: Chemical structure of valsartan.
Valsartan (VAL) (Figure 2) is a tetrazol–byphenil–valinic derivative of losartan, structurally characterized by the presence of a sole heterocyclic structure [13]. VAL has shown to be effective in decreasing blood pressure values and treating heart failure [14-15]. Methods such as HPLC [16-18], and simultaneous UV spectrophotometric methods [19-20] are reported for estimation of VAL alone or in combination with hypertensive agents. However, no method is available for simultaneous determination of amlodipine with valsartan by using box behnken design expert software for optimization.
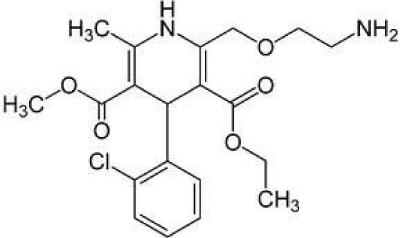
Figure 2: Chemical structure of amlodipine.
According to the information extracted from literature to date, there is not even a single method reported for the simultaneous determination of AML and VAL using Box Behnken factorial design in pharmaceutical formulations. The novelty of the present method includes the development of a newer solvent system using methanol: triethylamine buffer. The method was validated for linearity, accuracy, precision, LOD, LOQ, system suitability, and selectivity as per ICH guidelines [21]. Recently, the use of design of experiments (DOE) approach in analytical method development, termed as analytical DOE, has become quite popular in practice [21]. It is documented to provide risk-based understanding of the analytical as well as major factors affecting the performance of analytical method [22]. Based on the principles of design of experiments, it helps in thorough understanding of the plausible risk(s) and associated interaction(s) among the method variables, respectively [23]. It involves screening and method optimizing using experimental designs, optimum search through response surface methodology to embark upon the analytical design space, and postulating control strategy for continuous improvement [24].
In the past a few years, several literature reports have successfully demonstrated the immense applicability of DOE approach for developing efficient and cost-efficient LC methods for estimation of analytes separately and in combinations for bulk drugs, pharmaceutical formulations, respectively [25-28].
Attempts were, therefore, made to develop a straight, rapid, sensitive, robust, effective and economical HPLC method employing DOE approach for estimation of AML and VAL in bulk drug and pharmaceutical formulations. Furthermore, experimental design was used for optimization of mobile phase by taking methanol, pH and flow rate as variables and their effect was seen at a retention time of both the compounds that would serve as an assay method for combination drug product of AML and VAL.
Experimental
Chemicals and reagents
Amlodipine and Valsartan were received as gratis sample from Prudence pharma, Gujrat and Taj pharma, Gujrat. Methanol (HPLC grade) from Qualigen, orthophosphoric acid and triethylamine (AR grade) were purchased from E-Merck Ltd. (Mumbai, India). Ultrapurified HPLC grade water was obtained from the Milli - Q® system (Millipore, Milford, MA, USA) water purification unit. Mobile phase was filtered using 0.45μ nylon filters made by Millipore (USA) and was sonicated and degassed using sonicator.
HPLC instrumentation and chromatographic conditions
HPLC system of Waters, with UV detector was used for the separation drug. The system was empowered by compaq pressario and a rheodyne injection valve with a 20 μL loop was used for injection of the sample. A Hypersil C-18 column (250*4.6 mm, i.d., 5 μm particle size) was used. The mobile phase was composed of methanol: triethylamine buffer at pH 3.0, in the various ratios with a flow rate of 1.0ml/min. HPLC system was operated at room temperature (25 ± 2°C).
Preparation of standard solution
A calculated amount of 50 mg of AML and VAL was weighed and dissolved separately in methanol. The solution was sonicated for 15 minutes to completely dissolve both the components. Both stock solutions were mixed together and the volume was adjusted to 100ml. The stock solution was further diluted to obtain a final concentration of AML and VAL for estimation. The solution was filtered through 0.45μ nylon filters before analysis.
Calibration curve
Calibration curves were prepared by taking appropriate aliquots from AML and VAL stock solutions in volumetric flask and diluted up to the mark with mobile phase to obtain final concentrations of 5-50 μg/ml of AML and 10-100 μg/ml of VAL. The standard solutions were injected through the 20μl loop system and chromatograms were obtained using 1.0 ml/min flow rate and monitored at 237 nm. Calibration curve was constructed by plotting average peak area against the concentration and regression equation was computed.
Optimization
The optimization of mobile phase condition was performed as per the experimental design employing a three factor three level Box– Behnken design (BBD) using Design-Expert 8.0.5 software (Stat-Ease Inc., Minneapolis, USA) by selecting the methanol volume (X1), flow rate (X2), and pH (X3) as independent variables, while the retention time of AML (Y1), and retention time of VAL (Y2) as responses. Response surface analyses were carried out to identify the effect of different independent variables on the observed responses.
Table 1 illustrates total 15 experimental runs obtained from Box Behnken design with their observed responses and predicted responses. The responses were statistically evaluated using the ANOVA procedure. Further, the optimum condition was selected by the numerical optimization procedure using the desirability function. BBD has the advantage of optimization for experiments by using 3k-factorial design (where k=1, 2, 3 . . .) having at least three dependent variables or factors and more than one response as compared to other experimental designs such as central composite design (CCD) and fractional factorial design (FFD) [22-25]. The general polynomial equation quadratic model is
S.No
X1
methanol
X2
pH
X3
flow rate
Y1
(Rt-AML)
Y2
(Rt-VAL)
Actual Predicted
Actual Predicted
1
50
2
3.5
4.63
4.62
11.43
11.38
2
50
1.5
3
4.36
4.38
10.84
10.87
3
50
2
2.5
4.40
4.43
10.11
10.08
4
25
2
3
4.12
4.15
10.22
10.28
5
25
1
3
3.49
3.45
09.29
09.37
6
50
1
3.5
4.10
4.13
09.53
09.47
7
75
1
3
4.55
4.52
10.75
10.67
8
75
1.5
3.5
4.51
4.54
11.03
11.12
9
50
1.5
3
4.35
4.39
10.86
10.89
10
75
2
3
4.89
4.93
11.91
11.82
11
25
1.5
2.5
3.41
3.38
09.11
09.04
12
25
1.5
3.5
3.62
3.63
09.61
09.57
13
75
1.5
2.5
4.29
4.31
10.51
10.55
14
50
1.5
3
4.35
4.36
10.90
10.87
15
50
1
2.5
3.91
3.92
09.71
09.75
Table : Design matrix used for optimization of mobile phase condition with their obtained responses.
Y = β0 + β1 X1 + β2 X2 + β3 X3 +β12 X1X2 + β13 X1X3+β23 X2X3 +β11 X1 ²+β22 X2 ²+β33 X3 ² + · · ·
Where, Y is the measured response associated with each factor level combination; β0 is constant; β1, β2 , β3 are linear coefficients, β12, β13, β23 are interaction coefficients between the three factors, β11, β22, β33 are quadratic coefficients computed from the observed experimental values of Y from experimental runs and A, B and C are the coded levels of independent variables high (+), low (−) and center point (0). The terms AB and A2 represent the interaction and quadratic terms, respectively.
Validation
Linearity: The linearity of an analytical method is its ability to show a directly proportional relationship of a quantitative response to a specific concentration of analyte within a given specified range of concentrations. The linearity of both the compound has been made by serial dilution of the stock solution using the suitable aliquots to yield calibration curves over the concentration range of 5-50 μg/ml and 10-100 μg/ml for AML and VAL respectively. Three replicate analyses of each of the concentrations were used to establish the calibration curve.
Accuracy: Accuracy was determined by the injection of (n=5) of known concentrations of both drugs that had been prepared from new stock solutions. The measured concentrations of these samples were extrapolated from a calibration curve specifically generated for the determination of the accuracy of the method.
Precision: The precision of the proposed method was evaluated by carrying out five independent assays of AML and VAL over the concentration ranges studied. Intermediate precision was carried out by analyzing the samples by a different analyst on another instrument. %RSD of the all assays were obtained and calculated.
Recovery: Recovery of the method was determined by spiking the sample at three levels with 80%, 100% and 120% of standard solutions. These mixtures of both the compounds were analyzed by the proposed method. The experiment was performed and their recoveries and % RSD were calculated.
Selectivity: To check the selectivity of the proposed method, mixture of AML and VAL was prepared with tablet formulation. The comparison of its area with the area of the standard solution was done along with the percentage recovery of both the analytes.
Limit of quantitation (LOQ) and limit of detection (LOD): The parameters LOD and LOQ were determined on the basis of signal to noise ratio, LOD & LOQ was calculated by the method which was based on the standard deviation (SD) of the response and the slope (S) of the calibration curve at levels approximating the LOD & LOQ. LOD & LOQ were determined as follows.
LOD = 3.3 X Standard deviation of y intercept / Slope of calibration curve
LOQ = 10 X Standard deviation of y intercept / Slope of calibration curve
Robustness: As defined by the ICH, the robustness of an analytical procedure refers to its capability to remain unaffected by small and deliberate variations in method parameters [26]. The conditions studied were mobile phase composition (buffer±5%), wavelength (altered by ±2) and use of LC columns from different batches.
System suitability study: System suitability parameters were measured so as to verify the system performance. System precision was determined on six replicate injections of standard preparations. All important characteristics, including tailing factor and theoretical plate number were measured.
Application of the method to dosage forms: An average of in house developed ten tablets of AML and VALS were weighed and ground to fine powder. Accurately weighed powder sample equivalent to (containing 5mg of AML and 80mg of VAL) were dissolved in methanol. The flask was placed in an ultrasonic bath at room temperature for 10min. After sonication, the solution was allowed to stand for 5.0 min. and 1.0 ml of sample was diluted with methanol. The sample was filtered and 0.5μl of this solution was injected. The average content of the tablets was determined using the corresponding regression equation.
Results and Discussion
The suitability of mobile phase combination, flow rate, and pH was decided on the basis of linearity, sensitivity, system suitability, selectivity, lesser time required for analysis (low retention time), peak parameters. Out of several tried combinations as suggested by BBD, the mobile phase composition of methanol-triethylamine buffer showed efficient chromatographic separation of AML and VAL (10μg/mL) with retention time of 4.35 minutes and 10.26 minutes, respectively as shown in Figure 3. The use of methanol in method development than other organic solvents is a cost-effective approach for regular routine analysis of pharmaceutical formulations alone or in combination.
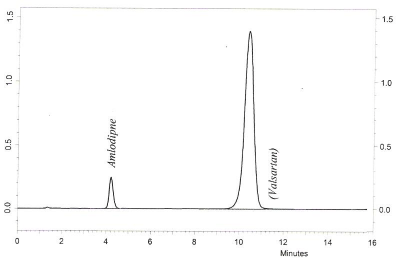
Figure 3: Chromatograms of amlodipine and valsartan reference samples.
Optimization of mobile phase
A total 15 compositions were prepared as per the experimental design and for resolution of peak and retention time for both the drugs as shown in Table 1. The response surface analysis was carried out to understand the effect of selected independent variables on the observed responses. The mathematical relationships were established and coefficients of the second order polynomial equation, generated using MLRA for retention time for AML and VAL were found to be quadratic in nature with interaction terms. The coefficients of the polynomials fit well to the data, with the values of R2 ranging between 0.9958 to 0.9997 for AML and 0.9914 to 0.9969 for VAL (p<0.05 in all the cases). Figure 4a depicts a response surface plot, characterizing increase in the retention time increased with increasing the concentration of methanol, whereas an increase in flow rate increase in retention time followed by a gradual decrease. Hence, it can be revealed that at the intermediate levels of flow rate the retention time was found to be optimized. Similarly, Figure 4b depicts a relationship between pH of the mobile phase and retention time of both drugs. It was observed that the increase in pH of mobile phase does not significantly affect the retention time. All the response surfaces were best fitted with quadratic polynomial models, and able to predict the interaction effects too. Finally, the model was observed for ANOVA (p<0.001), which revealed that the model terms for main effects and interaction effects were statistically significant. The ANOVA results are enumerated in Table 2. Finally, the optimized mobile phase condition was selected by numerical point prediction optimization method from the software having the desirability value as 1. The composition of the optimized condition was found to be methanol (60%), flow rate (1.0ml/min), pH (3) respectively.
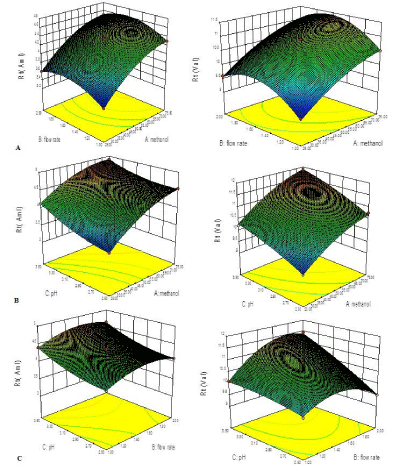
Figure 4: Three dimensional response surfaces (A) Effect of factor X1
(methanol %), Effect of factor X2 (flow rate), Effect of factor X3 (pH), on
response Y1 (Rt of AML) response Y2 (Rt of VAL).
ANOVA parameters
AML
VAL
Adjusted R2
0.9992
0.9914
Predicted R2
0.9958
0.9533
DF
3
3
F value
6.75
9.72
Prob>F
0.1318
0.0947
R2 value
0.9997
0.9969
Suggested model
Quadratic
Quadratic
Y1(Rt of Aml) = +4.35+0.45 X1+0.10 X2+0.25X3+0.032 X1X2-0.067X1X3+ 7.5 X2X3-0.20X12-0.21X22+0.11X32.
Y2(Rt of Val) = +10.87+0.74X1+0.27X2 +0.55X3+ 0.054X1X2 + 0.063X1X3+ 0.38 X2X3- 0.23X12-0.59X22-0.098X32.
Table 3: Optimized analytical regression parameters of Amlodipine and Valsartan.
Table 2: Summary analysis of ANOVA results of amlodipine and valsartan.
Linearity
The results of the validation procedure for linearity reveal that the above assay was linear over the concentration range 5-50μg/ml for AML and 10-100μg/ml for VAL. The regression coefficients were found to be 0.9977 for AML and 0.995 for VAL. The relevant equations for these are Y=350748x+ 14075 and Y= 492882x + 27042 for AML and VAL respectively, shown in Table 3. The test for linearity of the proposed analytical method yielded R2 values that were greater than 0.990 for both drugs used during validation.
Parameter
Amlodipine
Valsartan
Range (μg/ml)*
5-50
10-100
Retention time(min.)
4.35
10.26
Slope
350748
492882
Intercept
350748x+ 14075
492882x + 27042
Correlation coefficient
0.9977
0.995
Retention time(min)
10.6±0.003
4.35±0.005
LOD (μg/ml)
1.2
1.45
LOQ (μg/ml)
3.71
4.39
*Data represents the mean of 3 determinations.
Table 3: Optimized analytical regression parameters of Amlodipine and Valsartan.
Accuracy
The accuracy of the samples has been calculated from measured concentrations of these samples were extrapolated from a calibration curve specifically generated for the determination of the accuracy of the method. The results of accuracy studies for both the compounds AML and VAL are summarized in Table 4. It is clearly evident from the result that, %RSD of both compounds was found to be less than 2 hence the method can be considered accurate.
Drug
Level
(%)
Concentration
(µg/ml)
Amount recovered (µg/ml)
% Recovery
% RSD
Amlodipine*
80
7.95
7.89
99.24
0.72
100
10.14
9.96
101.8
1.17
120
12.08
11.97
99.08
0.46
Valsartan*
80
7.98
7.93
99.37
1.16
100
10.04
9.98
99.4
0.94
120
12.16
12.21
100.41
0.48
*Data represents the mean of 5 determinations.
Table 4: Accuracy at three level of amlodipine and valsartan by optimized HPLC method.
Precision
Precision was assessed by the measurement of inter-day precision by assay of three different concentrations of AML and VAL (10, 20 and 30μg/ml) at different time intervals in the different day and interday precision by repetition for same days. The RSD (%) for inter-day and intraday precision for AML were in the range of 0.342–0.527% and 0.378–0.875, respectively and for inter-day and intra-day of VAL had been found in the range of 0.268-0.822 and 0.528- 0.749 respectively, which were found to be within the acceptable limit. The method showed good precision for both drugs and data are summarized in Table 5.
Compound
Concentration
(µg/ml)
Interday precision
Mean %RSD
Intraday precision
Mean %RSD
Amlodipine*
10
9.91
0.342
10.05
0.378
20
19.97
0.376
19.85
0.458
30
31.55
0.527
30.02
0.875
Valsartan*
10
10.16
0.478
09.97
0.664
20
20.25
0.822
19.34
0.528
30
29.97
0.268
31.04
0.749
*Data represents the mean of 5 determinations.
Table 5: Interday and Intraday precision of amlodipine and valsartan by optimized HPLC method.
Specificity and selectivity
Specificity and selectivity were studied for the examination of the presence of interfering components in the working solution of AML and VAL. The results indicate that the retention time of AML and VAL is at 4.36 and 10.85 minutes, respectively. There is no variation in the retention time of the both the compounds as compared with the standard drug solution. They are free from interference from formulation excipients and solvent from each other. This indicates the method is selected and specific for determination AML and VAL simultaneously.
Limit of detection & Limit of quantification
The LOD and LOQ of AML were found to be 1.21 and 3.7μg/ ml, respectively, while for VAL were 1.45 and 4.39μg/ml, respectively. RSD (%) of six replicates injections of AML at LOD and LOQ were 7.31 and 4.16, respectively. Similarly % RSD of six replicates injections of VAL at LOD and LOQ were 8.54 and 3.61, respectively. The values (Table 3) indicated that the method was very sensitive to quantify both the drugs.
Robustness
In the developed RP-HPLC method, small deliberate variations in the optimized method parameters were done. The effect of change in wavelength and buffer concentration and use of LC columns from different batches, on the percent recovery of both the compounds was studied. The results showed that the slight variations in the chromatographic conditions used to study the effect have shown negligible variation on the retention time of both drugs showing the method is highly robust for its intended use.
System suitability study
The system suitability was assessed by taking six replicate of 10μg/ml concentration of AML and VAL and their capacity factor, retention time and area were determined for both drugs. The capacity factor for VAL and AML was found to be 2.62 and 7.58 min, which shows the both the compounds were suitable for the system. The % RSD for other parameters of both the compounds is not more than 2.0%. So results of system suitability parameters are in the acceptable limit of systems suitability parameters.
Application of the method to dosage forms
The developed HPLC method is sensitive and specific for the quantitative determination of AML and VAL. The method was validated for different parameters and, hence has been applied for the estimation of drug in pharmaceutical dosage forms. The in house developed tablets of were evaluated for the amount of drug present in the formulation. Each sample was analyzed in triplicate after extracting the drug as mentioned in the sample preparation of the experimental section. The recovered amount of AML and VAL were 98.20% and 99.82%, respectively Table 6. None of the tablet ingredients interfered with the analyte peak. The proposed method has used application of 33-factorial design using BBD for optimization of mobile phase for the simultaneous estimation AML and VAL showed that change in mobile phase combination has a direct effect on retention time. The method was validated for linearity, precision, accuracy, sensitivity, system suitability, and robustness were proved to be convenient and effective for the quality control as well as simultaneous routine analysis of AML and VAL in pharmaceutical dosage forms. The measured signal was shown to be precise, accurate, and linear over the concentration range tested with retention time of 4.35min and 10.26min makes it economical due to lower solvent consumption. The % RSD for all parameters was found to be less than two, which indicates the validity of method and assay results obtained by this method are in fair agreement. Also it can be utilized for determination of content uniformity and dissolution profiling of product, where sample load is higher and high throughput is essential for faster delivery of results.
Drug
Amount loaded
(mg)
Amount found
(mg)
%
Mean recovery
%
RSD
Amlodipine*
5
04.91
98.20
1.45
Valsartan*
80
79.86
99.82
0.90
*Data represents the mean of 5 determinations.
Table 6: Recovery analysis of Amlodipine and Valsartan in tablet formulation.
Conclusion
A simple, rapid, sensitive and economical analytical method has been successfully developed employing the systematic approach for quantification of AML and VAL in bulk drug as well as in house tablet formulations. The optimal setting of chromatographic conditions was in the analytical design space using the desirability function. Validation of the method corroborated excellent linearity, accuracy, precision, system suitability, specificity and robustness. Further, the experimentally observed values of LOD and LOQ of both drugs were also quite lower. The method demonstrated a high degree of practical utility for estimation of combination drugs in pharmaceutical dosage forms.
References
- Budavari S. The Merck index, Merck and Co. Press: Whitehouse Station, NJ, 12th Edn, 1997.
- Richard FD, Habibullah H, Klinke WP, Pierre D, Claude N, Denis CP, et al. Effect of amlodipine, atenolol and their combination on myocardial ischemia during treadmill exercise and ambulatory monitoring. J. Am. Coll. Cardiol. 1995; 25: 619- 625.
- John ED, Jean MD, Philippe S, Paul RL, Eric T, Jan B, et al. Medical treatment of myocardial ischemia in coronary artery disease: effect of drug regime and irregular dosing in the CAPE II trial. J. Am. Coll. Cardiol. 2002; 40: 917-925.
- Zarghi A, Foroutan SM, Shafaati A, Khoddam A. Validated HPLC method for determination of amlodipine in human plasma and its application to pharmacokinetic studies. Farmaco. 2005; 60: 789-792.
- Bahrami G, Mirzaeei SH. Simple and rapid HPLC method for determination of Amlodipine in human serum with fluorescence detection and its use in pharmacokinetic studies. J. Pharm. Biomed. Anal. 2004; 36: 163-168.
- Chaudhari B, Patel NM. Development and Validation of HPLC method for simultaneous estimation of Atorvastatin Calcium and Amlodipine Besylate. J. Pharm Res. 2006; 5: 141-144.
- Argekar AP, Powar SG. Simultaneous determination of atenolol and amlodipine in tablets by high-performance thin-layer chromatography. J Pharm Biomed Anal. 2000; 21: 1137-1142.
- Chaudhari BG, Patel NM, Sham PB. Stability Indicating RP-HPLC for simultaneous determination of Atorvastatin Calcium and Amlodipine Besylate from their combination drug products. Chem. Pharm. Bull. 2007; 55: 241-246.
- Naidu KR, Kale UN, Shingare MS. Stability indicating RP-HPLC method for simultaneous determination of amlodipine and benazepril hydrochloride from their combination drug product. J Pharm Biomed Anal. 2005; 39: 147-155.
- Ravi SS, Nanjan MJ, Vasudevan M, Shaat N, Suresh B, Sankar SR. Simultaneous estimation of atenolol and amlodipine in formulation by RP-HPLC. Ind. J. Pharm. Sci. 1997; 59: 171-173.
- Feng Y, Zhang L, Shen Z, Pan F, Zhang Z. Analysis of amlodipine in human plasma by liquid chromatography-mass spectrometry. J Chromatogr Sci. 2002; 40: 49-53.
- Bhatt J, Singh S, Subbaiah G, Shah B, Kambli S, Ameta S. A rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the estimation of amlodipine in human plasma. Biomed Chromatogr. 2007; 21: 169-175.
- Bauer JH, Reams GP. The angiotensin II type 1 receptor antagonists. A new class of antihypertensive drugs. Arch Intern Med. 1995; 155: 1361-1368.
- Kim S, Yoshiyama M, Izumi Y, Kawano H, Kimoto M, Zhan Y, et al. Effects of combination of ACE inhibitor and angiotensin receptor blocker on cardiac remodeling, cardiac function, and survival in rat heart failure. Circulation. 2001; 103: 148-154.
- Palatini P, Malacco E, Fogari R, Carretta R, Bonaduce D, Bertocchi F, et al. A multicenter randomized double-blind study of valsartan/hydrochlorothiazide combination versus amlodipine in patients with mild to moderate hypertension. J. Hypertens. 2001; 19: 1691– 1696.
- Daneshtalab N, Lewanczuk RZ, Jamali F. High performance liquid chromatographic analysis of angiotensin II receptor antagonist Valsartan using a liquid extraction method. J. Chromat. B Analy. Technol. Biomed. Life Sci. 2002; 766: 345-359.
- Gonzalez L, Lopez JA, Alonso RM, Jimenez RM. Fast screening method for the determination of angiotensin II receptor antagonists in human plasma by high-performance liquid chromatography with fluorimetric detection. J Chromat. A. 2002; 949: 49-60.
- Li H, Wang Y, Jiang Y, Tang Y, Wang J, Zhao L, et al. A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of valsartan and hydrochlorothiazide in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 852: 436-442.
- Satana E, Altimay S, Goger GN, Sibel A, Ozkan SA. Simultaneous determination of Valsartan and Hydrochlorothiazide in tablets by first-derivative ultraviolet spectrophotometry and LC. J Pharm Biomed Anal. 2001; 25: 1009-1013.
- Tatar S, Sağlìk S. Comparison of UV- and second derivative-spectrophotometric and LC methods for the determination of valsartan in pharmaceutical formulation. J Pharm Biomed Anal. 2002; 30: 371-375.
- Monks K, Molnár I, Rieger HJ, Bogáti B, Szabó E. Quality by Design: Multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J Chromatogr A. 2012; 1232: 218-230.
- Rozet E, Ziemons E, Marini RD, Boulanger B, Hubert P. Quality by design compliant analytical method validation. Anal Chem. 2012; 84: 106-112.
- Nethercote P, Ermer J. Quality by design for analytical methods: implications for method validation and transfer. Pharmaceutical Technology. 2012; 36: 74–79.
- Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J. 2008; 10: 268-276.
- Garg LK, Reddy VS, Sait SS, Krishnamurthy T, Vali SJ, Reddy AM. Quality by design: design of experiments approach prior to the validation of a stability-indicating HPLC method for montelukast. Chromatographia. 2013; 76: 1697–1706.
- Beg S, Kohli K, Swain S, Hasnain MS. Development and validation of RP-HPLC method for quantitation of amoxicillin trihydrate in bulk and pharmaceutical formulations using Box-Behnken experimental design. Journal of Liquid Chromatography and Related Technologies. 2011; 35: 393–406.
- Imam SS, Aqil M, Akhtar M, Sultana Y, Ali A. Optimization of mobile phase by 32-mixture design for the validation and quantification of risperidone in bulk and pharmaceutical formulations using RP-HPLC. Analytical Methods. 2013: 6: 222–228.
- Kurmi M, Kumar S, Singh B, Singh S. Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide. J Pharm Biomed Anal. 2014; 96: 135-143.
- ICH (Q2R1). Draft Guidelines on Validation of Analytical Procedures: Text and Methodology, IFPMA: Switzerland, 1995.