
Special Article – Instrumental Analysis
Austin J Anal Pharm Chem. 2016; 3(2): 1066.
Validated Stability-Indicating Spectrophotometric Techniques for the Determination of Quetiapine in the Presence of its Oxidation-Induced Degradation Products
Youssef RM1*, Barary MA1, Abdine HH1 and Wagih MM2
1Faculty of Pharmacy, Department of Pharmaceutical Analytical Chemistry, University of Alexandria, El- Messalah, Alexandria 21521, Egypt
2Forensic Medicine Authority, Egyptian Ministry of Justice,Biram El Tonsy Street, El Sayada Zeinab, Zanhom 11628, Cairo, Egypt
*Corresponding author: Rasha M Youssef, Faculty of Pharmacy, Department of Pharmaceutical Analytical Chemistry, University of Alexandria, El-Messalah, Alexandria 21521, Egypt
Received: May 21, 2016; Accepted: June 09, 2016; Published: June 13, 2016
Abstract
The International Conference on Harmonization (ICH) guideline entitled “Stability Testing of New Drug Substances and Products” requires that stress testing be carried out to elucidate the inherent stability characteristics of the active substance. Susceptibility to oxidation is one of the required tests. Also, evaluation of the hydrolytic and the photolytic stability is required.
Four new selective, precise, and accurate methods are described for the determination of Quetiapine (QTI) in the presence of its oxidation-induced degradation products in both the raw material and pharmaceutical preparations.
Method A and B is based on third-derivative (3D), and fourth-derivative (4D) spectrophotometric measurement of QTI in methanolic solution at the zerocrossing point of its oxidation-induced degradation products (at 272–290, and 260–281 nm, respectively).
Method C is a 1DD spectrophotometric method based on the simultaneous use of the first derivative of the ratio spectra and the measurement of peak amplitude at 250- 277 nm.
Method D uses a pH-induced absorbance-difference spectrophotometry with (ΔA) measurement at 217 nm.
These methods are suitable as stability-indicating for the determination of QTI in the presence of its oxidation-induced degradation products either in the bulk powder or in pharmaceutical preparations.
Keywords: Quetiapine; Oxidation-induced degradation products; Raw material and pharmaceutical preparations; Validated stability-indicating methods
Introduction
Quetiapine (QTI) is one of the most recent “atypical” antipsychotic drugs, whose use in schizophrenia is becoming widespread. QTI and the active human plasma metabolite, N-desalkyl quetiapine interact with a broad range of neurotransmitter receptors. QTI and N-desalkyl QTI exhibit affinity for brain serotonin (5HT2) and dopamine D1 and D2 receptors. QTI and N-desalkyl QTI also have high affinity at histaminergic and adrenergic alpha1 receptors, with a lower affinity at adrenergic alpha2 receptors [1,2].
Until now, official pharmacopoeias have not reported any monograph or official method for assay of QTI. Only some HPLC methods [2-6] and voltammetric method [7] have been developed for determination of QTI in biological fluids. These described methods use solid phase extraction (SPE) or liquid–liquid extraction (LLE) to deal with plasma samples with a lower limit of quantification.
The structure of QTI (Figure 1) shows that it contains a sulfur atom in thiazine ring and tertiary amino groups which are susceptible to oxidation [8] (Figure 2). The purpose of the work described in this paper was to develop simple, selective, and accurate methods for the determination of QTI in the presence of its oxidation-induced degradation products in the raw material and in pharmaceutical preparations. The spectrophotometric methods have the advantages of speed, low cost, and protection of the environment.
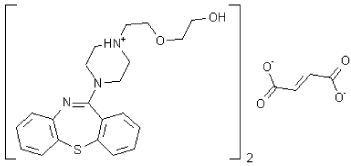
Figure 1: Structure of intact Quetiapine hemi fumerate.
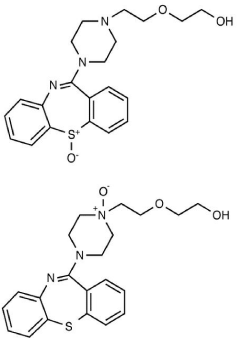
Figure 2: Oxidation-induced degradation products of Quetiapine.
Experimental
Instrumentation
All spectrophotometric measurements were made by using a Perkin-Elmer Lambda EZ 201 UV-visible spectrophotometer (Waltham, MA). The absorption spectra of test and reference solutions in 1 cm matched quartz cells were recorded over the range 200–400 nm.
Materials and Reagents
Pharmaceutical grade QTI was kindly supplied by Pharaonia Pharmaceuticals (Alexandria, Egypt) and was certified to contain 99.9% QTI. Methanol, chloroform, and hydrogen peroxide (50%, v/v), were analytical grade. The commercial QTI tablets used (Batch No. 499) were produced by Hi-Pharm (Cairo, Egypt); each tablet contained 200 mg QTI.
Preparation of reference standard solutions
Standard solutions of QTI (60 mg%) were prepared in methanol for the spectrophotometric methods and in methanol for its determination by methods A, B, C and D.
Preparation of oxidation-induced degradation products
An accurately weighed 40 mg QTI was dissolved in 25 mL methanol. Subsequently, 4 mL hydrogen peroxide (50%, v/v) was added, and the solution was heated in a boiling water bath for 20 min and then alkalinization using 1N NaOH was done and heated again in a boiling water bath for 15 min for the oxidation and the removal of excess hydrogen peroxide. The solution is neutralized using 1N HCl and quantitatively completed to volume with methanol.
The course of the degradation was investigated by TLC using silica gel 60 F250 TLC plates and a mobile phase of chloroform: methanol (9:1, v/v). After 15min, a single peak with an Rf value of 0.4 was obtained, but no peak was observed at an Rf value of 0.84, corresponding to intact QTI indicating complete degradation of the drug. The solution was quantitatively transferred to a 50-mL volumetric flask and dilution was made to volume with methanol.
Construction of calibration graphs
Method A and B: Aliquots from the standard solution of QTI in methanol, within concentration range stated in Table 1, were accurately transferred into a set of 10-mL volumetric flasks. Dilution was made to volume with methanol and the absorption spectrum for each solution was recorded against methanol as a blank.
The 3D (method A) and 4D (method B) spectra (obtained by using Δλ= 17 nm) were recorded versus the blank. The values of the 3D and 4D amplitudes were measured from peak to peak at 272–290 and 260– 281 nm, respectively, and plotted versus the concentrations of QTI to obtain a linear relationship.
Method C: Aliquots from the standard solution of QTI in methanol, within concentration range stated in Table 1, were accurately transferred into a set of 10-mL volumetric flasks. The absorption spectra of these solutions were divided by the “divisor” (oxidation- induced degradation products at 0.8 μg/mL); the ratio spectra were obtained were smooth, and the first derivatives of the ratio spectra (1DD) were recorded by using Δλ= 9 nm).
The values of 1DD amplitudes were measured from peak to peak at (250–277) nm and were found proportional to the concentrations of QTI. Calibration graph for QTI was constructed by plotting the 1DD values versus concentration and the regression equation was computed.
Method D: Into two sets of 10-mL volumetric flasks, equal portions from standard solution of QTI in methanol, within the concentration range cited in Table 1, were transferred. The volume of each flask in both sets was completed to 2mL with methanol. Then, the contents of the flasks in one set were diluted to volume with 0.1N HCl, and the contents of the flasks in the other set were diluted to volume with 0.1N NaOH.
The (ΔA) spectra of the drug solutions in 0.1NHCl were recorded versus the corresponding solutions in 0.1NNaOH. The values of the ΔA amplitudes were measured at 217±2 nm and plotted versus the concentrations of QTI to obtain a linear relationship.
Results and Discussion
The purpose of this work was to develop simple and sensitive stability-indicating techniques for the determination of QTI in the presence of its oxidation- induced degradation products.
Method A and B
Third-derivative (3D), and fourth-derivative (4D) spectrophotometric measurement of QTI in methanolic solution at the zero-crossing point of its oxidation- induced degradation products at 272–290, and 260–281 nm, respectively (Figure 3,4,5). The main instrumental parameter affecting the shape of derivative spectra is the wavelength increment over which the derivative spectrum is obtained (Δλ). This parameter needs to be optimized to obtain a well-resolved large peak, good selectivity, and increased sensitivity for the determination [9]. Generally, the noise level decreases with an increase in Δλ, thus decreasing the fluctuation in the derivative spectrum. However, if the value of Δλ is too large, the spectral resolution is very poor. Therefore, the optimum value of Δλ should be determined by taking into account the noise level and the resolution of the spectrum. Some values of Δλ were tested and Δλ = 17 nm was selected for the 3D and 4D spectrophotometric method as the optimal condition to give a satisfactory signal-to-noise ratio.
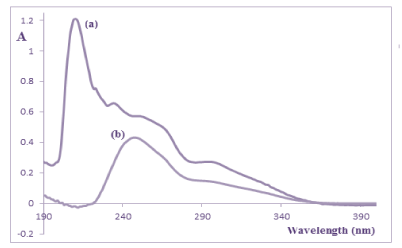
Figure 3: UV absorption spectra of (a) 14μg/mL QTI and (b) 5μg/mL of its
oxidation-induced degradation products in methanolic solutions.
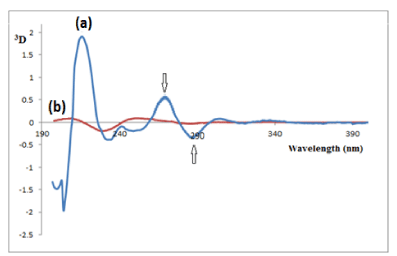
Figure 4: Third-derivative (3D) of (a) 20μg/mL QTI and (b) 2μg/mL of its
oxidation-induced degradation products, both in a methanolic solution.
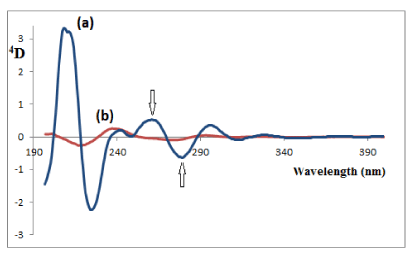
Figure 5: Fourth-derivative (4D) of (a) 20μg/mL QTI and (b) 2μg/mL of its
oxidation-induced degradation products, both in a methanolic solution.
In general, the characteristics profiles of the derivative spectra may constitute a specific fingerprint useful for drug identification. In particular, the ratios of the amplitudes at selected wavelengths can be regarded as suitable parameters for confirmation of identity, purity, and stability of the drug. The maximum value of 3D272/3D290 for QTI was found to be 0.742 with a relative standard deviation (RSD) of 0.725% (n = 5), and the maximum value for 4D260/4D281 for QTI was found to be 0.953 with an RSD of 1.43% (n = 5).
Method C (1DD Method)
1D spectrophotometric method based on the simultaneous use of the first derivative of the ratio spectra and the measurement of peak amplitude at 250- 277 nm. The main advantage of the 1DD method may be the opportunity to measure corresponding peaks; thus, there is the potential for greater sensitivity and accuracy. The main disadvantages of the zero-crossing method are the risk of small drifts in the working wavelengths and the fact that the working wavelengths generally do not produce corresponding peaks in the derivative spectrum [10].
To optimize the 1DD method for the determination of QTI in the presence of its oxidation- induced degradation products, it is necessary to test the influence of two variables: divisor concentration and Δλ. Both variables were studied (Table.2, 3). The influence of the Δλ in obtaining the 1DD was tested, and Δλ = 9 nm was selected as the optimum value. A correct choice of divisor concentration is fundamental for several reasons. For example, in the wavelength range where the absorbance of the spectrum used as the divisor is zero or below the baseline, the noise of the ratio spectra is greatly increased. Therefore, a certain overlap of the spectra in the region of the working wavelength is actually desirable to avoid an increase in the error. If the concentration of the divisor is increased or decreased, the resulting values of the derivative ratio are proportionally decreased or increased with consequent variation in both the sensitivity and the range of linearity. In this method, the UV absorption spectra of QTI were divided by the concentration of the oxidation- induced degradation products, 0.8μg/mL. The first order was calculated for the ratio spectra obtained with Δλ = 9 nm (Figure 6). The concentration of QTI was proportional to the 1DD amplitude at 250-277 nm over the concentration range shown in Table 1.
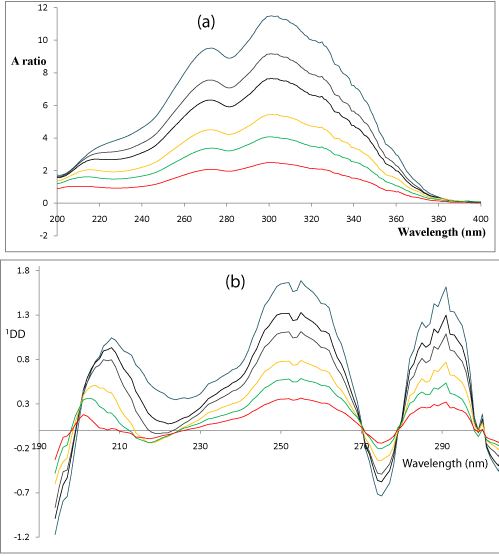
Figure 6: (a) Absorption ratio and (b) first-derivative of the absorption ratio
(1DD) spectra obtained for different concentrations (6, 10, 14, 20, 24 and
30μg/mL) of QTI by using the oxidation-induced degradation products at
0.8μg/mL as a divisor and Δλ= 9 nm.
Method D (ΔA Method) (pH-induced absorbance-difference spectrophotometry with ΔA measurement at 217 nm)
The application of difference spectrophotometry is very advantageous both in quantitative and qualitative analysis. The main goal of this method is to increase the selectivity of the measurement by eliminating the effect of foreign light absorption or the interference of other substances on the absorption of the analyte, if the spectra of these substances do not change with the use of different pH values or different solvents [11] (as in the present case).
The absorbance-difference spectra (ΔA) between the acidic solution and the alkaline solution of QTI and between the corresponding solutions of the oxidation- induced degradation products were recorded; from the spectral characteristics, it is clear that the absorbance-difference spectrophotometry with (ΔA) measurement at 217 nm can be used for the selective determination of intact QTI in the presence of its; ΔA at 217nm for the latter reads zero (Figure 7).
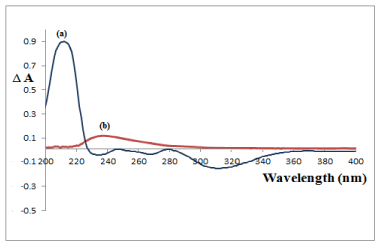
Figure 7: Absorbance-difference spectra (ΔA) spectra of (a) 14μg/mL QTI
and (b) 1.2μg/mL of its oxidation-induced degradation products in acidic
solution versus alkaline solution.
Validation of the proposed methods
Validation of the proposed methods were carried out according to the ICH guidelines [12].
Linearity: The linearity of the proposed methods was evaluated by analyzing a series of different concentrations of QTI. According to the ICH, 5 concentrations must be used. Under the experimental conditions described above, the calibration graphs of the spectrophotometric peak amplitudes at the specified wavelengths versus certain QTI concentration ranges (Table 1) are linear.
Slopes, intercepts, correlation coefficients, standard deviations of the slope, and intercept (obtained by the linear least-squares treatment of the results) are also given in Table 1. The values for the correlation coefficients were not sufficient to evaluate the linearity of the calibration graphs.
Therefore, linearity was evaluated by calculation of the RSD of the slope. Also, the small degree of scatter in the experimental data points around the lines of regression was confirmed by the small values of the variances around the slopes, Sb2 [13]. For additional confirmation, the Student’s t-test was performed to determine whether the experimental intercept (a) of the above-mentioned regression lines was not significantly different from the null hypothesis. The calculated values of t (a/Sa) do not exceed the 95% criterion of t = 2.31 for 5 samples. Therefore, the intercepts are not significantly different from zero in the spectrophotometric methods. Thus, the hypothesis that (a) is of negligible value was confirmed.
Accuracy and precision: The accuracy study was performed by adding known amounts of QTI and its oxidation-induced degradation products to known concentrations of the commercial capsules (standard additions method). The resulting mixtures were assayed, and the results obtained for QTI were compared with the expected results. The excellent recoveries for the standard additions method suggest good accuracy of the proposed methods and no interference from either the oxidation-induced degradation products of QTI or the capsule’s excipients; thus, the proposed methods were found to be applicable to the determination of QTI in its dosage form.
Intraday precision of the proposed methods was evaluated by assaying freshly prepared solutions at 3 different concentrations within 1 day. Interday precision was evaluated by assaying freshly prepared solutions at 3 different concentrations on 3 different days.
Detection and quantitation limits: According to the ICH recommendations, the approach based on the standard deviation of the response and the slope was used to determine the detection and quantitation limits. The theoretical values were assessed practically and are given in Table 1.
Selectivity: The selectivity of the methods was evaluated by preparing different mixtures of QTI within the linear concentration range and of its oxidation-induced degradation products; the ratio of QTI to its oxidation-induced degradation products. The synthetic mixtures were analyzed according to the procedures described for the proposed methods. Satisfactory results were obtained, indicating the high selectivity of the proposed stability-indicating methods for the determination of QTI.
Conclusion
The proposed methods are simple to use and provide accurate and reproducible results for the determination of QTI in pharmaceutical tablets, without any interference from excipients or the oxidationinduced degradation products. Compared with the official stabilityindicating HPLC method, the spectrophotometric methods have advantages of low cost, rapid measurement, and protection of the environment.
Acknowledgement
The authors would like to thank central laboratory of Alexandria University. Authors are also thankful to digital library of Faculty of Pharmacy-Alexandria University for providing documentations and references.
References
- Friedman JH. Atypical antipsychotics in the EPS-vulnerable patient. Psychoneuroendocrinology. 2003; 28 Suppl 1: 39-51.
- Mandrioli R, Fanali S, Ferranti A, Raggi MA. HPLC analysis of the novel antipsychotic drug quetiapine in human plasma. J Pharm Biomed Anal. 2002; 30: 969-977.
- Hasselstrøm J, Linnet K. Fully automated on-line quantification of quetiapine in human serum by solid phase extraction and liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 798: 9-16.
- Pan RN, Kuo BP, Pao LH. Validated LC-MS-MS method for the determination of quetiapine in human plasma: application to a pharmacokinetic study. J Chromatogr Sci. 2012; 50: 277-282.
- Saracino MA, Mercolini L, Flotta G, Albers LJ, Merli R, Raggi MA. Simultaneous Determination of Fluvoxamine isomers and Quetiapine in human plasma by means of High Performance Liquid Chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 2: 227-233.
- Davis PC, Wong J, Gefvert O. Analysis and pharmacokinetics of quetiapine and two metabolites in human plasma using reversed-phase HPLC with ultraviolet and electrochemical detection. J Pharm Biomed Anal. 1999; 20: 271-282.
- Sibel A, Dogan B, Uslu B. Voltammetric analysis of the novel atypical antipsychotic drug quetiapine in human serum and urine. Microchim. Act. 2010; 153: 27-35.
- Trivedi RK, Patel MC. Development and validation of a stability indicating RPUPLC method for determination of quetiapine in pharmaceutical dosage form. Sci Pharm. 2011; 79: 97-111.
- El- Gindy A, El-Zeany B, Awad T, and Shabana MM. Derivative spectrophotometric, thin layer chromatographic-densitometric and high performance liquid chromatographic determination of trifluoperazine hydrochloride in presence of its hydrogen peroxide induced-degradation product. J. Pharm. Biomed. Anal. 2002; 27: 9-18.
- Morelli B. Simultaneous determination of ceftriaxone and streptomycin in mixture by ratio-spectra 2nd derivative and zero-crossing 3rd derivative spectrophotometry. Talanta, 1994; 41: 673-683.
- Maghgoub H, Gazy AA, El-Yazbi FA, El-Sayed MA and Youssef RM. Spectrophotometric determination of binary mixtures of pseudoephedrine with some histamine H1 – receptor antagonists using ratio spectrum method. J. Pharm. Biomed. Anal, 2003; 31: 801-809.
- Alvarenga L, Ferreira D, Altekruse D, Menezes JC, Lochmann D. Tablet identification using near-infrared spectroscopy (NIRS) for pharmaceutical quality control. J Pharm Biomed Anal. 2008; 48: 62-69.
- Miller JN and Miller JC. “Statistics and Chemometrics for Analytical Chemistry”, 4th Ed., Prentice Hall, Harlow, England, 2000; 283-285.