
Research Article
Austin J Anal Pharm Chem. 2017; 4(1): 1080.
Rapid and Reliable Determination of Glipizide in Pharmaceutical Samples by HPLC and Its Degradation Study
Basavaiah K1* and Rajendraprasad N2
¹Department of Chemistry, University of Mysore, Manasagangothri, Mysuru-570 006, Karnataka, India
²PG Department of Chemistry, JSS College of Arts, Commerce & Science, B N Road, Mysuru-570 025, Karnataka, India
*Corresponding author: Basavaiah K, Department of Chemistry, University of Mysore, Manasagangothri, Mysuru-570 006, Karnataka, India
Received: January 08, 2017; Accepted: February 08, 2017; Published: February 10, 2017
Abstract
A simple, specific and rapid high-performance liquid chromatographic (HPLC) method for the determination of glipizide (GPZ) in pharmaceutical sample is described. Reversed phase chromatography was performed on an Inertsil ODS 3V (150 × 4.6mm; 5μm particle size) column with an isocratic mobile phase consisting of 10mM potassium dihydrogen phosphate (pH 3.9) and methanol (60:40 v/v). The effluent was monitored on a uv detector at 220nm and the column temperature was maintained at 35°C. Linear response (r=0.9999) was observed over the range, 1-450μgmL-1; and the limits of detection (LOD) and quantification (LOQ) were calculated to be 0.03 and 0.09 μgmL-1, respectively. Both intra-day and inter-day precisions at three tested concentrations were excellent, with %RSD values of <1% and the accuracy was better than 1.5% (RE). The method was also validated for robustness, ruggedness and selectivity and the results were satisfactory. The method was applied to the determination of GPZ in tablets and the results agreed well with the label claim and those obtained by European Pharmacopeial method. Entire assay was complete in less than 10min. As part of degradation study, the drug was subjected to acid-, base-, peroxide-, heat-, and light-induced stress conditions, and the drug was found to be susceptible to degradation under oxidation, and inert to other conditions.
Introduction
Glipizide (GPZ), chemically known as N-[2[4[[[(cyclohexylamino)carbonyl]amino] sulfonyl] phenyl] ethyl]-5- methylpyrazinecarboxamide (Figure 1) is an oral antihyper glycaemic agent [1]. It belongs to sulphonyl-urea class of anti-diabetics, which are indicated for type-2 diabetes mellitus [2]. The drug functions by stimulating the pancreas to release insulin by closing the ATP-dependent potassium channels in the β-cell membrane, which leads to an opening of the calcium channels. The resulting influx of calcium induces insulin secretion.
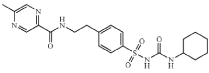
Figure 1: Structure of GPZ.
Owing to its therapeutic importance different methods are available for determination of GPZ in body fluids and include, HPLC [3-11], LC-MS/MS [12-16], UPLC-MS/MS [17,18] and radioimmunoassay [19]. European Pharmacopeia [20] describes a titrimetric assay in which drug in dimethyl formamide is titrated with lithium methoxide using quinaldine red as indicator. Methods based on numerous other techniques have been suggested for the determination of GPZ in pharmaceuticals including, uv-spectrophotometry [21-24], UPLC [25,26], TLC [27,28], HPTLC [29] and voltammetry [30].
HPLC offers many advantages over other techniques, including minimum sample manipulation prior to chromatography, rapid analysis, and simultaneous determination of multiple compounds with good accuracy, precision and selectivity. Literature survey revealed the availability of few HPLC methods for GPZ. An RP-HPLC method was described by Vijaya et al [31] for the determination of GPZ in dosage forms using C18 column (250 × 4.6 mm; 5 μm). The mobile phase consisted of methanol- triethylamine buffer, pH 3 (35: 65) with a flow rate of 1mLmin-1 and UV- detection at 230nm. Linearity was found in the range, 0.1-10μgmL-1. Mantri and Shanmukhappa [32] developed and validated an RP-HPLC method with C18 analytical column using methanol- 0.115% w/v ammonium hydrogenphosphate buffer pumped at 1mLmin-1 at ambient temperature. The calibration graph was linear in the range of 10-70 μg mL-1. Rapid and sensitive assay of GPZ was achieved by Rahila and Asif [33]. The drug was chromatographed on a RP- C18 column with mobile phase consisting of 0.05M KH2PO4, pH 7.0- methanol (15: 85 v/v) pumped at a flow rate of 1mLmin-1. Quantification was achieved by monitoring the UV absorbance at 225nm. The method showed linearity in the range 10-2000 ngmL-1. An Inertsil ODS-C18 column (250 × 4.6 mm; 5μm) in isocratic mode with mobile phase containing methanol– water- 0.01M KH2PO4, (70: 25: 5 v/v/v) at a flow rate of 1.5 mLmin-1 and UV- detection at 270nm was used by Rayanam et al [34] for the assay of GPZ in tablet formulation. The method has been found to be sensitive with LOD and LOQ values of 15 and 45 μgmL-1, respectively. The method was also applied for blood serum. Determination of GPZ in sustainedrelease tablets by RP-HPLC has been reported by Liping et al. [35] Separation and assay were carried out on an Eclipse XDB- C18 column with 0.1M NaH2PO4-methanol (54: 46) as mobile phase pumped at a flow rate of 1mLmin-1 and UV detection at 225nm. The linear range was from 5 to 250 μgmL-1 GPZ.
Apart from the above methods for GPZ in single component dosage forms,several workers have applied HPLC for the simultaneous determination of GPZ inmulti-component dosage forms when the drug is present along with glimepiride [36], metformin [37-39], simvastatin [40], pioglitazone and rosiglitazone [41], rosiglitazone, pioglitazone, glibenclamide andglimepiride [42], metformin, pioglitazone, glimeperide, gliclazide andglibenclamide [43], metformin, pioglitazone, phenformin, gliclazide, glimeperide,gl ibenclamidetolbutamide, rosiglitazone and pioglitazone [44].
The reported HPLC methods for single component tablets [31-35] suffer from low detection limits and narrow linear dynamic ranges of applicability, and none of them is stability-indicating. The stability study should be undertaken to elucidate the intrinsic stability of the drug substance. Stability testing of a drug substance should be carried out under different stress conditions, such as hydrolysis, oxidation, heat and light to evaluate the stability-indicating supremacy of an analytical method used for assay [45,46]. Driven by the need for an HPLC method, which is sensitive with a wide linear dynamic range, and also stability-indicating, this study was taken up. This work describes the development and validation of a rapid and reliable HPLC method for the determination of GPZ in bulk drug and tablets containing only GPZ (single-component). The method was found to be stability indicating.
Materials and Methods
Instrument and software
Chromatographic analysis was performed with a Waters HPLC system (Waters Corporation, Milford, USA) equipped with Alliances 2695 series low pressure quaternary gradient pump, a programmable variable wavelength UV detector and auto sampler. Data were collected and processed using Waters Empower 2 software.
Reagents
HPLC grade methanol was purchased from Merck, Mumbai, India; potassium dihydrogen orthophosphate, triethylamine, orthophosphoricacid, hydrochloric acid, sodium hydroxide and hydrogen peroxide were from Qualigens Ltd., India. Water purified by the Milli-Q system (Millipore, Milford, Massachusetts, USA) was used to prepare mobile phase. A 10mM potassium dihydrogen orthophosphate solution was prepared by dissolving required quantity of salt in 1000mL water and pH adjusted to 3.9 using triethylamine or dilute phosphoric acid. A 600mL portion of this buffer was mixed with 400mL of methanol (60:40 v/v), shaken well and filtered using 0.45μm Nylon membrane filter. Hydrochloric acid (HCl, 2M) and hydrogen peroxide (H2O2, 5%) were prepared by appropriate dilution, and sodium hydroxide (NaOH, 2M) was prepared by dissolving calculated amount of the chemical in water.
Materials
Working standard of GPZ was a generic gift from Bal Pharma, Bangalore, India. Dibizide-5 and Glynase-5 tablets each containing 5mg active content, were purchased from local market.
Chromatographic conditions
Analysis was isocratic at 0.8mlmin-1 flow rate with 10mM KHPO4 buffer: methanol (60:40) as mobile phase. The mobile phase was premixed, filtered through 0.2μm membrane filter to remove any particulate matter and degassed before use. Chromatographic separation was achieved on an Inertsil ODS 3V (150mm × 4.6mm, 5μm particle size) column. The detector wavelength was set at 220nm and the injection volume was 20μL. The column temperature was maintained at 35°C. Prior to injecting solutions, the column was equilibrated for at least 30min with the mobile phase flowing through the system.
Standard GPZ solution
Accurately weighed 100mg of pure GPZ was dissolved in and diluted with the mobile phase to the mark in a 100mL volumetric flask to get 1000μgmL-1 GPZ stock solution.
General procedures
Procedure for bulk drug: Procedure for preparation of calibration curve: Working standard solutions containing 1-450 μgmL-1 GPZ were prepared by serial dilution of stock solution. An aliquot of 20μL was injected (three injections) and eluted with the mobile phase under the stated chromatographic conditions. A plot of average peak area versus concentration was prepared. Alternatively, the regression equation was derived using mean peak area-concentration data and the concentration of the unknown was computed from the regression equation.
Procedure for tablets: Tablet powder equivalent to 100mg GPZ was transferred into a 100mL calibrated flask containing 60mL of mobile phase. The mixture was sonicated for 20min to achieve complete dissolution of GPZ, and the content was then diluted to volume with the same solvent to yield a concentration of 1000μgmL- 1 GPZ, and filtered through a 0.2μm nylon membrane filter. The tablet extract was injected on to the HPLC column in replicates, after dilution to 300μgmL-1 level.
Procedure for placebo blank and synthetic mixture: A placebo blank was prepared by homogeneous mixing of hydroxyl cellulose (10mg), acacia (15mg), starch (10mg), sodium citrate (15mg), magnesium stearate (15mg), talc (15mg) and sodium alginate (10mg). A 100mg of the placebo blank was taken, its solution prepared as described under ‘procedure for tablets’, and then subjected to chromatography by following the general procedure. A synthetic mixture was prepared by homogeneous mixing of an accurately weighed 100mg of pure GPZ with 100mg of placebo. A solution of synthetic mixture equivalent to 1000μgmL-1GPZ was prepared as described under “procedure for tablets”. The resulting solution was assayed (n= 5) by the proposed method after dilution to 300μgmL-1 GPZ with the mobile phase.
Procedure for stress study: A 3mL aliquot of 1000μgmL-1 GPZ solution was transferred into three different 10mL calibrated flasks and added 2ml of 2M HCl, 2ml 2M NaOH or 2ml 5% H2O2. The flasks were placed in a water bath maintained at 80°C for 2h. After cooling to room temperature acid/base was neutralised with 2ml 2M NaOH (acid hydrolysis) or 2ml 2M HCl (base hydrolysis), and diluted to the mark with the mobile phase. Each solution was separately chromatographed. To study behaviour to heat, solid sample kept in a Petri Dish was placed in an oven at 100°C for 24h. For photodegradation, solid sample was exposed to uv radiation of wavelength 254nm of 1200K lux intensity flux for 48h in an uv-chamber. Postexposure to heat and light, 300μgmL-1 solutions were prepared separately in the mobile phase and chromatographed.
Results and Discussion
To obtain good linearity, sensitivity and selectivity, the method was optimized and validated in accordance with the current ICH guidelines [47]. The typical chromatograms obtained for blank and pure GPZ under optimized HPLC conditions are depicted in Figure 2.

Figure 2: Chromatograms for: a) Blank (mobile phase) b) Pure GPZ solution
(300μgmL-1).
Method development
A well defined symmetrical peak and satisfactory results were obtained upon measuring the response of eluent under the optimized conditions after thorough experimental trials that could be summarized as follows:
Choice of column: For performance characteristics, columns including Chromatopack (250mm × 4.6mm, 5μm particle size); Hypersil BDS C8 (250mm × 4.0mm, 5.0μm particle size) thermo; Inertsil ODS 3V (250mm × 4.0mm, 5.0μm particle size); Luna C18 (250mm × 4.0mm, 5.0μm particle size) and Zorbax XDB (250mm × 4.0mm, 5.0μm particle size) were tried. The results revealed that the Inertsil ODS 3V column was more suitable since it gave better sensitivity.
Choice of wavelength: The UV detector response of GPZ was studied and the best wavelength was found to be 220 nm showing the highest sensitivity.
Mobile phase composition: Several modifications in the mobile phase composition were tried to obtain a symmetrical peak with a reasonable retention time. These modifications included type and proportion of the organic modifier, pH, strength of the phosphate buffer, and flow rate. The results shown in Table 1 reveal that highest number of theoretical plates resulted when the mobile phase consisted of phosphate buffer (pH 3.9 adjusted with 0.1% H3PO4) and methanol in the ratio of 60:40 and pumped at a flow rate of 0.8mlmin-1. Of several organic modifiers tried, methanol was found to give an elegent and highly sensitive peak.
Ratio
(A/B)a
Number
of
theoretical
plates (N)
pH of
the
medium
Number
of
theoretical
plates (N)
%
H3PO4
Number
of
theoretical
plates (N)
Flow
rate,
mL
min-1
Number
of
theoretical
plates (N)
40/60
50/50
55/45
60/40
70/30
-
-
4934
6588
7693
9261
8756
-
-
2.0
2.5
3.0
3.9
4.1
4.3
4.5
5789
7866
8679
9956
9867
8954
5724
0.050
0.075
0.100
0.125
0.150
0.200
0.250
8537
9649
9987
9657
8586
8246
8097
0.50
0.60
0.70
0.80
0.90
1.00
1.20
6176
7648
8765
9960
9768
9326
8976
aA- phosphate buffer and B- methanol.
Table 1: Effect of ratio of buffer to organic modifier, pH and ionic strength of buffer, and mobile phase flow rate on the number of theoretical plates.
Ratio of organic modifier: The effect of proportion of organic modifier on the sensitivity and retention time was investigated using mobile phases containing up to 30-60% methanol. Table 1 shows that 40% methanol was the best, giving well defined peak and the highest number of theoretical plates.
Effect of pH and ionic strength of buffer: The effect of pH of the mobile phase on the sensitivity and retention timeof the test solute was investigated using mobile phases of pH ranging from 2.0-4.5. The results (Table 1) revealed that pH 3.9 was the most appropriate, giving well defined peak and the highest number of theoretical plates. At lowerand higher pH, non-symmetrical peak and smaller number of theoretical plateswere obtained. Therefore pH 3.9 was fixed as optimum. The same trend wasobserved after making alteration in the ionic strength of the buffer and 10mM phosphate buffer was used as working buffer throughout the investigation.
The effect of flow rate: The effect of flow rate on the symmetry, sensitivity and retention time ofthe peak was studied, and a flow rate of 0.8mLmin-1 was optimal for bettersymmetry and reasonable retention time as shown in Table 1.
Method validation
Linearity: To study the linearity, standard solutions in the range 1 to 450 μgmL-1 GPZ were chromate graphed in triplicate and mean peak area for each standard calculated. A calibration graph was prepared by plotting mean peakarea against concentration, and linear relationship was established by least-square regression analysis. The calibration plot was linear over the concentration range, 1-450μg mL-1 (n= 3) (Figure 3) and can be described by the equation: y = m x + b
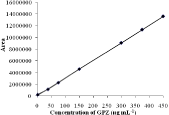
Figure 3: Calibration curve.
where y the mean peak area, x the concentration of GPZ in μgmL-1, m slope and b intercept. The slope (m), y-intercept (b) and theirstandard deviations were evaluated and are presented in Table 2. These results confirm the linear relationship between peak area and concentration aswell as the sensitivity of the method.
Parameter
Value
Linear range, μgmL-1
Limits of detection, (LOD), μgmL-1
Limits of quantification, (LOQ), μgmL-1
Regression equation, y*
Slope (m)
Intercept (b)
Standard deviation of intercept (Sb)
Standard deviation of slope (Sm)
Correlation coefficient (r)
1 -450
0.03
0.09
29955
86386
897.9
1681.2
0.9999
*y=mx+b, where y is the mean peak area, x concentration in μg mL-1, b intercept, m slope.
Table 2: Linearity and regression parameters.
Limits of quantification (LOQ) and detection (LOD): The limit of quantification (LOQ) was determined by establishing the lowest concentration that can be measured according to ICH recommendations [47], below which the calibration graph is non linear and was found to be 0.09μgmL-1. The limit of detection (LOD) was determined by establishing the minimum level at which the analyte can be reliably detected and it was found to be 0.03μgmL-1.
Precision and accuracy: Both repeatability (within-day precision) and reproducibility (between-day precision) was determined as follows:
Pure drug solution at three concentration levels (within the linear range) were prepared and injected in seven replicates during the same day for repeatability, and over a period of five days (five injections per day) for reproducibility. Percent RSD and %RE were calculated to judge the precision and accuracy. The results of this study compiled in Table 3 speak of excellent precision and accuracy of the proposed method.
GPZ
injected,
μg mL-1
Intra-day
Inter-day
GPZ found,
μg mL-1
%
REa
%
RSDb
%
RSDc
GPZ found,
μg mL-1
%
REa
%
RSDb
%
RSDc
150
300
450
151.8
298.7
448.2
1.20
0.43
0.40
0.63
0.45
0.54
0.59
0.36
0.47
152.1
297.5
452.7
1.40
0.83
0.60
0.49
0.34
0.61
0.27
0.31
0.95
aRelative error; bRelative standard deviation based on peak area; cRelative standard deviation based on retention time.
Table 3: Results of accuracy and precision study (n=5).
Method robustness: The robustness of the method was evaluated by making small but deliberate changes in the chromatographic conditions. The chromatographic conditions varied were: flow rate (0.8 and 0.8±0.1 mL), wavelength (220 and 220±1 nm), temperature (35 and 35±2°C) and mobile phase composition (60 and 60±5% buffer: 40 and 40±5% methanol). These slight alterations did not significantly affect the system suitability parameters: retention time, tailing factor and number of theoretical plates as indicated by low values of %RSD (Table 4) (measure of intermediate precision).
Condition
altered
Modification
Mean peak
area
± SD*
%
RSD
Mean Rt
± SD*
%
RSD
Mean
theoretical
plates
± SD*
%
RSD
Mean
tailing
factor
±SD*
%
RSD
Actual
-
9084249
± 55609
0.61
5.583±
0.003
0.054
9946±
5.727
0.06
1.258
±0.004
0.32
Column
temperature
33?C
35?C
37?C
9126732
± 91333
1.00
5.612±
0.002
0.036
9889±
6.724
0.07
1.213
±0.005
0.41
Mobile
phase
composition
(Buffer:
methanol)
55:45
60:40
65:35
9164999
± 98404
1.07
5.492±
0.003
0.055
9935±
8.114
0.08
1.227
±0.003
0.25
Flow rate
0.7 mL min-1
0.8 mL min-1
0.9 mL min-1
9136736
± 97336
1.06
5.590±
0.002
0.036
9892±
4.772
0.05
1.221
±0.004
0.33
Wavelength
219 nm
220 nm
221 nm
9126632
± 91313
1.00
5.572±
0.003
0.054
9954±
3.663
0.04
1.211
±0.005
0.41
*Mean value of three determinations at GPZ concentration of 300μgmL-1.
Table 4: Results of method robustness.
Method ruggedness
The ruggedness of the method was assessed by comparison of the results for the assay of GPZ performed by three analysts in the same laboratory. The inter-personal RSD did not exceed 1.07% indicating the ruggedness of the method (Table 5).
Variable
Mean
Peak
area
± SD*
%RSD
Mean
Rt ±
SD*
%RSD
Mean
theoretical
plates
±SD
%RSD
Mean
tailing
factor±
SD*
%RSD
Analysts
(n=3)
9164999
± 98404
1.07
5.592±
0.003
0.054
9899±
7.614
0.08
1.221±
0.003
0.25
*Mean value of three determinations at GPZ concentration of 300μgmL-1.
Table 5: Results of method ruggedness (n=3).
Selectivity
Selectivity of the method was evaluated by injecting the mobile phase, placebo blank, pure drug solution and tablet extract. No peaks were observed for mobile phase and placebo blank, and no extra peaks were observed for tablet extract (Figure 4). Synthetic mixture solution, when analysed at 300μgmL-1 concentration level, yielded percent recoveries of 97.36±1.2 indicating the absence of interference from the tablet excipients.
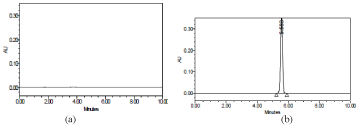
Figure 4: Chromatograms obtained for: a) placebo blank and b) tablet extract
(300μgmL-1 GPZ).
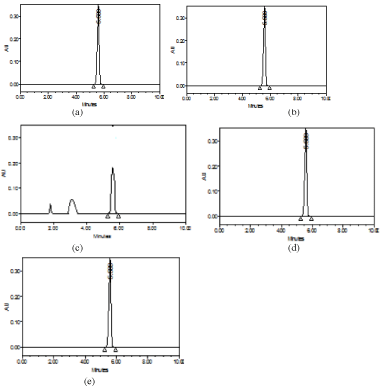
Figure 5: Chromatograms of GPZ (300μgmL-1) after forced degradation:
a) acid degradation; b) base degradation; c) peroxide degradation; d) thermal
degradation and e) photolytic degradation.
Solution stability
The drug solution was injected at different time intervals of 0, 12 and 24 h, and chromatograms were recorded. At the specified time interval, %RSD value for peak area, retention time and number of theoretical plates was calculated, and subsequently pooled %RSD was computed. This value, which is <1% (Table 6), amply demonstrates that the crucial system suitability parameters remained unaltered indicating the stability of the solution over a period of 24h.
Time, h
Mean
Peak
area
± SD*
Pooled %RSD
Mean
Rt ±
SD*
Pooled %RSD
Mean
theoretical
plates
±SD
Pooled %RSD
Mean
tailing
factor±
SD*
Pooled %RSD
0
9084249
± 55609
0.92
5.592±
0.002
0.04
9885±
8.565
0.10
1.258±
0.004
0.33
12
9126732
± 91333
5.581±
0.002
9989±
9.774
1.213±
0.005
24
9164999
± 98404
5.603±
0.003
9835±
9.524
1.227±
0.003
*Mean value of three determinations for GPZ concentration of 300μgmL-1 at each time interval.
Table 6: Results of solution stability.
Application to tablets
The developed method was applied to the determination of GPZ in two brands of tablets containing 5mg GPZ per tablet. Quantification was performed using the regression equation. The same tablet powder was used for assay by official method (20) for comparison. The results were compared statistically by applying the Student’s test for accuracy and F-test for precision. As shown by the results compiled in Table 7, the calculated t-test and F-values did not exceed the tabulated values of 2.77 and 6.39 for four degrees of freedom at the 95% confidence level, suggesting that the proposed method and the reference method do not differ significantly with respect to accuracy and precision.
Tablet
brand
name
Nominal
amount,
mg
NTG found* (%) ± SD
t-value
F- value
Reference
method
Proposed
method
Dibizide
5
98.67±0.87
99.48±0.56
1.75
2.41
Glynase
5
99.45±1.10
100.5±0.42
1.61
1.34
*Mean value of five determinations. Tabulated t-value at 95% confidence level is 2.77; Tabulated F-value at 95% confidence level is 6.39.
Table 7: Results of determination of GPZ in tablet and statistical comparison with the reference method.
Accuracy by recovery test
The accuracy of the proposed method was further checked by performing recovery test. Pre-analyzed tablet powder was spiked with pure GPZ at three different concentration levels and the total was determined by the proposed method. Each determination was repeated three times. The recovery of pure drug added was quantitative (Table 8) and revealed that co-formulated substances did not interfere in the determination.
Tablet
studied
NTG in
tablet,
µg mL-1
Pure NTG
added,
µg mL-1
Total
found,
µg mL-1
Pure NTG
recovered*
(%NTG ±SD)
Dibizide
99.48
99.48
99.48
50
100
150
150.2
198.7
252.5
100.5±0.97
99.63±0.87
101.2±1.05
Glynase
100.5
100.5
100.5
50
100
150
148.6
199.5
251.5
98.75±0.67
99.49±0.65
100.4±0.90
Mean value of three determinations.
Table 8: Results of recovery study by standard addition method.
Results of forced degradation study
All forced degradation experiments were performed at 300μgmL- 1 concentration level. The inference with regard vulnerability to degradation was based on the comparison of the chromatograms recorded post-degradation with those obtained under optimized chromatographic conditions. GPZ was found to be more stable under photolytic and thermal stress conditions in solid state. The drug was also stable towards acid and base hydrolysis at elevated temperature. The drug degraded up to 60.9% under oxidative stress condition. The chromatograms obtained for GPZ after subjecting to forced degradation are presented in Figure 5. The results of this study are given in Table 9.
Degradation condition
% Degradation
Acid hydrolysis (2M HCl, 80?C, 2h)
No degradation
Base hydrolysis (2M NaOH, 80?C, 2h)
No degradation
Oxidation (5% H2O2, 80?C, 2h)
60.9%
Thermal (105 �C, 3 hours)
No degradation
Photolytic (1.2 million lux hours)
No degradation
Table 9: Results of degradation study.
Conclusions
This is the first article describing the stability-indicating reversed phase HPLC determination of glipizide in pharmaceutical samples whenever it is present alone. The method has been determined to be a good alternative to several HPLC methods reported earlier with respect to linear range and sensitivity. Shorter run time (<10min) and low flow rate (0.8mlmin-1) make the method cost-effective interms of overall analysis time and consumption of mobile phase. The method has been shown to be both precise and accurate, besides being both robust and rugged, and may be useful in routine analysis.
Acknowledgement
Authors thank Bal Pharma, Bangalore, India, for gifting glipizide pure sample. Prof. K. Basavaiah gratefully acknowledges the financial assistance by UGC, New Delhi, India, in the form of BSR Faculty fellowship.
References
- Sweetman SC. Martindale: The Complete Drug Reference, 34th Ed., Pharmaceutical Press, London, 2005; 5083.
- Brogden RN, Heel RC, Pakes GE, and Speight TM. Glipizide: a review of its pharmacological properties and therapeutic use. Drugs. 1979; 18: 329-353.
- Haakan E. High-performance liquid chromatographic determination of glipizide in human plasma and urine. J. Chromatogr B: Biomed. Sci. App. 1987; 421: 319-326.
- Lakshmi KS and Rajesh T. Separation and Quantification of Eight Antidiabetic Drugs on A High-Performance Liquid Chromatography: Its Application to Human Plasma Assay. ISRN pharmaceutics. 2011; 2011: 1-7.
- Rahila SJ, Manisha PP, Prabha MR, Asif K and Pramod YG. Sensitive and selective analytical method for the quantification of glipizide in human plasma. Int. Res. J. Pharm. 2010; 1: 378-383.
- Swaroop LR, Prashanth PK, Ashish HA and Rajesh NB. Development and validation of RP-HPLC method for analysis of glipizide in guinea pig plasma and its application to pharmacokinetic study. Int. J. Pharm Tech Res. 2010; 2: 1649-1654.
- Emilsson H. High-performance liquid chromatographic determination of glipizide in human plasma and urine. J. Chromatogr. 1987; 421: 319-326.
- Bae JW, Kim NT, Choi C, Kim MJ, Jang CG and Lee SY. HPLC analysis of plasma glipizide and its application to pharmacokinetic study. J. Liquid Chromatogr. Rel. Tech. 2009; 32: 1969-1977.
- Venkatesh P, Harisudhan T, Choudhury H, Mullangi R and Srinivas NR. Simultaneous estimation of six anti-diabetic drugs--glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomed. Chromatogr. 2006; 20: 1043-1048.
- Aburuz S, Millership J and McElnay J. The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma. J Chromatogr B: Anal. Tech. Biomed. Life Sci. 2005; 817: 277-286.
- Yang L, Lijuan H, Jide W, Chunrui D, Shiru X, Feng S, et al. Simultaneous determination of three diabetic drugs in urine by high performance liquid chromatography. Fenxi Ceshi Xuebao. 2011; 30: 293-297.
- Ning L, Ying D, Feng Q, Jia Y and Famei L. A rapid and reliable UPLC-MS/MS method for the identification and quantification of fourteen synthetic anti-diabetic drugs in adulterated Chinese proprietary medicines and dietary supplements. Biomed. Chromatogr. 2010; 24: 1255-1261.
- Atif M, Khalid SH, Onn GLK, Sulaiman SA, Asif M and Chandrasekaran A. Development and validation of RP-HPLC-UV method for the determination of Glipizide in human plasma. J. Young Pharm. 2013; 5: 26-29.
- Li N, Deng Y, Qin F, Yu J and Li F. Simultaneous quantification of metformin and glipizide in human plasma by high-performance liquid chromatography–tandem mass spectrometry and its application to a pharmacokinetic study. Biomed. Chromatogr. 2013; 27: 191-196.
- Ding CG, Zhou Z, Ge QH, Zhi XJ and Ma LL. Simultaneous determination of metformin and glipizide in human plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2007; 21: 132-138.
- Lin ZJ, Krieger DD and Shum L. Simultaneous determination of glipizide and rosiglitazone unbound drug concentrations in plasma by equilibrium dialysis and liquid chromatography-tandem mass spectrometry. J. Chromatogr B: Anal. Tech. Biomed. Life Sci. 2004; 801: 265-272.
- Qiu X, Zheng S, Wang Y, Wang R and Ye L. A rapid and simple UPLC-MS-MS method for determination of glipizide in human plasma and its application to bioequivalence study. J. Chromatogr. Sci. 2015; 53: 85-89.
- Ying ZC, Ling XJ, Guo LZ and Qiang LN. UPLC/MS/MS determination of glyburide and glipizide added in traditional Chinese medicinal preparation. Chinese J. Pharm. Anal. 2008; 28: 326-328.
- Maggi E, Pianezzola P and Valzelli G. Radioimmunoassay of glipizide in human plasma. European J. Clin. Pharm. 1981; 21: 251-255.
- European Pharmacopoea 6.0, Official Monograph 01/2008:0906, p. 1977.
- Aruna A and Nancey K. Simultaneous estimation of metformin HCL and glipizide in solid dosage forms by ultraviolet spectrophotometry. Indian Drugs. 2000; 37: 533-536.
- Chungath TT, Reddy YP and Devanna N. Simultaneous spectrophotometric estimation of metformin hydrochloride and glipizide in tablet dosage forms. Int. J. Pharm Tech Res. 2011; 3: 2064-2067.
- Sarangi RR, Panda SN, Panda SK and Sahu KC. Simultaneous UV-Spectrophotometric Estimation of Glipizide and Metformin in Bulk and Its Dosage Form. Int. J. Pharm. Biol. Arch. 2011; 2: 1137-1145.
- Adhikari L, Jagadev S, Sahoo S, Murthy PN and Mishra US. Devlopement and validation of UV-visible spectrophotometric method for simultaneous determination of pioglitazone hydrochloride, metformin hydrochloride and glipizide in its bulk and pharmaceutical dosage form. Int. J. Chem Tech Res. 2012; 4: 625-630.
- Trivedi HK, Kshtri N, Patel V and Roa V. Development and validation of an UPLC method for in-vitro study of glipizide extended release tablets. Int. J. Pharm. Sci. Res. 2012; 3: 3317-3322.
- Cijo MX, Basavaiah K, Vinay KB and Swamy N. Quality by design approach for the development and validation of glipizide, an antidiabetic drug, by RP-UPLC with application to formulated forms and urine. ISRN Chromatography. 2013; 2013 Article ID 738397: 1-10.
- El Kousy NM. Stability-indicating densitometric determination of some antidiabetic drugs in dosage forms, using TLC. Microchim. Acta. 1998; 128: 65-68.
- Gumienicze A, and Berecka A. Quantitative analysis of gliclazide and glipizide in tablets by a new validated and stability-indicating RPTLC method. J. Planar Chromatogr. Modern TLC. 2010; 23: 129-133.
- Modi DK and Patel BH. Simultaneous determination of metformin hydrochloride and glipizide in tablet formulation by HPTLC. J. Liq. Chromatogr. Rel. Tech. 2012; 35: 28-39.
- Ghoneim EM, El-Attar MA, Hammam E and Khashaba PY. Stripping voltammetric quantification of the anti-diabetic drug glipizide in bulk form and pharmaceutical formulation. J. Pharm. Biomed. Anal. 2007; 43: 1465-1469.
- Vijaya SS, Satya KV, Hima BV and Devala RG. High pressure liquid chromatography estimation of glipizide in pharmaceutical dosage forms. Asian J. Chem. 2006; 18: 1309-1312.
- Mantri MA and Shanmukhappa S. A validated RP-HPLC method for estimation of glipizide in its pure and pharmaceutical dosage form (tablets). Material Science Research India. 2009; 6: 223.
- Rahila S and Asif K. Reverse phase high performance liquid chromatographic method for the analysis of glipizide in pharmaceutical dosage forms. Int. J. Res. Ayur. Pharm. 2010; 1: 455-458.
- Rayanam VI, Rao LA and Ramana MV. Development and validation of LC method for the estimation of glipizide in pharmaceutical dosage form and serum. Int. J. Res. Pharm. Chem. 2011; 1: 50-54.
- Liping H, Yan X, Jiaxiu H, Jingping S, Jiali Z, Jin Z, et al. Determination of glipizide in sustained release tablets by RP-HPLC. Zhonghua Yixue Yanjiu Zazhi. 2011; 11: 337-339.
- Najma S, Arayne SM, Nadir AS and Hashim ZM. Simultaneous determination of glipizide and glimepride by Rp-Hplc in dosage formulations and in human serum. Med. Chem. Res. 2012; 21: 2443-2448.
- Jing F, Jia L, Dan S, Hua DR, Shun BK and Hui CX. Determination of two components in metformin and glipizide tablets by reversed-phase ion-pair HPLC. Chinese J. New Drugs Clin. Rem. 2010; 29: 445-447.
- Anurag D and Shukla IC. Simultaneous determination of glipizide and metformin hydrochloride in pharmaceutical preparation by HPLC. J. Indian Chem. Soc. 2004; 81: 84-86.
- Rao BU and Nikalje AP. Determination of glipizide, glibenlamide and glimeperide in a tablet dosage form in the presence of metformin hydrochloride by ion pair –reversed phase liquid chromatographic technique. J. Anal. Bioanal. Tech. 2010; 1: 1-5.
- Bhavana K and Srinivas M. RP-HPLC method for the simultaneous estimation of glipizide and simvastatin in bulk and in a synthetic mixture. Inv. Imp. Pharm. Anal. Quality Assur. 2015; 2015: 19-25.
- Anna G, Anna B and Lukasz K. Stability-indicating validated HPLC method for simultaneous determination of oral antidiabetic drugs from thiazolidinedione and sulfonylurea groups in combined dosage forms. J. AOAC Int. 2010; 93: 1086-1092.
- Lakshmi KS and Rajesh T. Development and validation of RP-HPLC method for simultaneous determination of glipizide, rosiglitazone, pioglitazone, glibenclamide and glimepiride in pharmaceutical dosage forms and human plasma. J. Iranian Chem. Soc. 2011; 8: 31-37.
- Reddy MU, Reddy PV, Somasekhar P and Varaprasad B. Single and high resolution RP-HPLC method for the determination of six anti diabetic drug products. J. Pharm. Res. 2011; 4: 1209-1212.
- Meng L and Subi L. Ion-pair HPLC determation of anti-diabetic agents in traditional Chinese medicines and health care products. Chinese J. Pharm. Anal. 2010; 30: 1038-1041.
- ICH Harmonized tripartite guideline Q1A (R2), Stability testing of new drug substances and products, Geneva, February, 2003.
- International Conference on Hormonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Stability Testing: Photostability Testing of New Drug Substances and Products, Q1B, November, 1996.
- International Conference on Hormonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (R1), Complementary Guideline on Methodology dated 06 November 1996, incorporated in November 2005, London. UK.