Abstract
Background: Adherence to imatinib in Chronic Myeloid Leukemia (CML) leads to optimal cytogenetic and molecular responses. Studies on this subject in Western countries have shown various results. The Glivec (imatinib) International Patient Assistance Program has been distributing at no cost the imatinib in 28 African countries. Cytogenetic and molecular responses to imatinib in patients eligible for this program remain poorer than expected. Non-adherence to imatinib is surveyed. The aim of this study is to assess the adherence and therapeutic persistence of imatinib in patients with chronic myeloid leukemia in Congo.
Patients and Methods: We studied imatinib adherence among 52 chronic myeloid leukemia patients with an average of 36.6 years (range 14 and 62 years) diagnosed at the chronic phase. The adhesion was evaluated through 3 different indirect methods:
1. Patients interview using the Morisky questionnaire (MMAS)
2. MPR
3. Discontinuation
The survival curve was determined for each group of patients: adherent and non adherent.
Results: Twenty-nine patients (55.76%) reported adherence to imatinib (MMAS>6), while the calculation of the number of imatinib capsules provided indicated that only 25% of patients were actually adherent (MPR>85%). Nonadherence according to MPR was associated with adolescent age (p = 0.002) and duration of treatment (p = 0.01). Progression of the disease was observed in 23 patients (43.23%). of these, 18 (78.3%) were non-adherent to treatment. Twenty-three patients discontinued treatment (43.23%). The average duration of interruption was 99 days. The average survival time at 60 months was 38% for the non-adherent group versus 82% for the adherent group.
Conclusion: Adherence to imatinib appears to be low. Different cultural, socio-economic, clinical and therapeutic factors are involved in non-adherence to the imatinib. They need to be identified in order to implement strategies to accompany patients.
Keywords: Adherence; Imatinib; Chronic myeloid leukemia; Congo
Introduction
Patient adherence to recommended treatment regimen leads to a good quality outcome. Despite this fact the patient’s adherence remains debatable. Adherence is poor, especially when the drug is taken orally [1]. Imatinib is an oral cancer therapy that has yielded impressive cytogenetic and molecular responses in CML [2]. It is a specific inhibitor of the tyrosine kinase that has demonstrated a significant activity in all phases of the Myeloid Chronic Leukemia (CML) [3]. Suboptimal responses are linked to high adherence to imatinib. However, the degree to which patients adhere to the imatinib is varies. Studies on the topic reported disparate results that range from 14 to 97% [4-9].
In the Sub Saharan region, the question of adherence to imatinib is unknown. Since early 2002, 28 African countries are eligible for the imatinib compassionate program that provides the drug at no cost. Consequently, imatinib is the first line treatment of CML in these countries. However, despite free, sustainable access to the drug by patients eligible for the compassionate program, reports on outcome and cytogenetic responses showed low performance [10-13]. We have made the hypothesis that poor outcome of patients under imatinib is linked to poor adherence to the treatment. Therefore, we report through this first study carried out in the African region, the adherence of imatinib to patients with CML in the Congo.
Material and Methods
Study design
GIPAP was implemented in the department of Hematology which is a tertiary medical facility that manages and monitors patients with CML since 2005. All patients with CML that was documented by cytogenetic studies are supplied at no cost with imatinib. Records of CML patients from 2008 to 2016 were reviewed to calculate the Overall Survival (OS) of adherent and non-adherent patients. OS is defined as the time elapsed between the imatinib initiations to death due to any cause.
From June 2015 to June 2016, we interviewed once each patient on imatinib 400 mg daily for at least one month to assess adherence to the imatinib and calculated the imatinib possession ratio and discontinuation delay during this time frame. Hematological response is monitored monthly during the first six months and then depending on the response every two or three months. The cytogenetic response was monitored at 6 months of treatment. For each Chronic Phase (CP) CML patient under imatinib for at least three months with a 400 mg daily dosage, we collected socio-demographic and clinical data from the medical record.
The study was approved the ethic committee of Brazzaville. All participants gave their written consents before participating in the study.
Data collection
Adherence: Adherence to imatinib was assessed by three indirect collection methods:
1. Questionnaire: Patients were interviewed individually by one hematologist of the department of Hematology. A questionnaire adapted from the eight item medication adherence scale by Morisky et al. was submitted to patients [14]. The interview was lead in French or local dialects depending on the participant’s choice. The Morisky et al. questionnaire includes 8 items that identify the barrier to non adherence. The 8 items consist of seven questions with yes and no answers and one item (the last one) with a 5-point Liker scale. Each item measures a specific medication taking behavior. MMAS score ranges from 0 to 8 and is classified in three levels. Score of 8 indicates high adherence, medium adherence between 6-7.75 and score <6 low adherence [14]. In this study, non adherence was defined by MMAS-8 inferior to 6 (75%) and adherence MMAS-8 = 6.
2. Medication Ratio Possession (MPR): MPR is the ratio between the number of days during which imatinib was acquired divided by the number of days between the first and the last acquisition of the imatinib. We calculated the MPR by dividing the number of imatinib possession by 365 days X 100. According to previous research studies on imatinib adherence a MPR of 85% was considered a midpoint threshold [7,15]. Consequently; patients were considered adherent to imatinib if they maintained an average of 85% or higher; they were considered non adherent when the MPR was inferior to 85%.
3. Discontinuation: Is defined as the gap period noticed without medication possession or the time during which the patient remains untreated for no medical reason. Discontinuation was reported when it was a 90 days or more gap [15]. It was measured by counting the total of days between the last refill and the date when the patient showed up at the Hematology Unit for a new refill. Patients for whom no gap was noticed where defined as persistent.
Statistical analysis
For the description of each quantitative variable, the average, range, frequencies and percentages were calculated. Odds Ratio (OR) and their 95% are presented for all quantitative variables. Fisher test was performed to study the patient’s adherence and factors influencing it. A significance level of p=<0.05 was considered. Analysis survival was calculated using the Kaplan-Meier method.
Results
Socio demographic data
52 patients were included in the study. They were 34 male (65.4%) and 18 female (34.6%).The mean age was 36.6 years (extreme 164and 62years). The characteristics of the patients are shown in Table 1.
Characteristics
N(%)
Sex
Male
34 (65.4)
Female
18 (34.6)
Total
52
Age at diagnoses (years)
Median
Range
36.6
14 – 62
Marital status
Single
Married
Divorced
5
3
45
Level of education
Without
Primary
Secondary
College
4
1
33
14
Time since diagnosis (months)
Median
Range
11.4
0.5-50
Time for follow-up (months)
Median
Range
28
3-88
Table 1: Characteristics of the patients with CML under imatinib.
Adherence
1. Questionnaire: The overall mean MMAS-8 was 5.78 (extreme 3-8). Twenty nine, 29 (55.76%) declared to be adherents to the imatinib and scored 6 or higher. Among them 4 (13.79%) scored 8, 17 (58.62%) scored 7 and 8 (27.58%) scored 6. Twenty three (44.23) scored below 6 and were characterized as no adherents.
2. MPR: The overall mean MPR was 71.30% (extreme 23 and 100%). Thirteen patients (25 %) were adherents with a MPR over 85%. Among them, 10 (52.6%) were aged over 40 years and 3 (10%) between 14-40 years old of the 52 participants, 39 (75%) were not adherents with a MPR that was ranging from 23-89%. Characteristics of the patients and outcomes according to their adherence per MPR are shown in Table 2.
Characteristics
Total(n=52)
Adherence
OR Brute(IC 95%)
P-value
No (n=39)75%
Yes (n=13)25%
MPR (mean)
Range
Age (%)
<16 years old
16-40 years old
>40 years old
71.30%
23-100%
3(5.8)
30(57.7)
19(36.54)
MPR<85%
3(100)
27(90.0)
9(47.4)
MPR>85%
0(0.0)
3(10.0)
10(52.6)
Baseline
10.3 (2.37-44.73)
0.002
Gender (%)
Female
Male
18(34.6)
34(65.4)
13(72.2)
26(76.5)
5(27.8)
8(23.5)
Baseline
0.8(0.21-2.93)
0.737
Duration of imatinib in month
Range
Cytogenetic response (%)
6th month
Major
Complete
Partial
Minor
No response
72.76
3-98
21(40.4)
2(9.5)
3(14.3)
10(47.6)
4(19.1)
2(9.5)
43.03
3-98
18(95.7)
0(0.0)
3(16.7)
10(55.6)
3(16.7)
2(11.1)
38.75
14-86
3 (4.3)
2(66.7)
0(0.0)
0(0.0)
1(33.3)
0(0.0)
Discontinuation(%)
11(22.0)
7(63.6)
4(36.4)
1.90 (0.45-8.01)
0.379
Progression of the disease (%)
23(48.9)
18(78.3)
5(21.7)
0.55 (0.15-2.04)
0.377
Table 2: Characteristics of the patients according their adherence status using the MPR.
Discontinuation
Twenty three patients over 52 (44.23%) discontinued their treatment. The overall period discontinuation period was 99.21 days (range 92 and 100).
Outcome
In the Kaplan Meier plot the mean overall survival time was 57.17 months. Adherent patients demonstrated a 5 year OS rate of 84% and 38% for the non adherent group Figure 1.
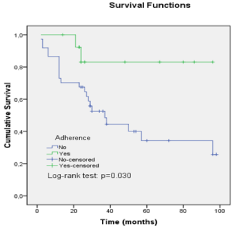
Figure 1: Overall survival of patients with CML adherent and non adherent
to imatinib therapy.
Discussion
Adherence to imatinib in the CML is the key to achieve major molecular and cytogenetic responses. The CML Advocate Network adherence survey mainly done in Europe reported that 33% of participants could be classified as highly adherent while 37% and 21% were respectively in the medium and low adherence group [16]. In Sub-Saharan the question is unexplored.
The poorest outcome of patients with cancer in developing countries is due to the lack of adequate health facilities, shortage of trained personnel but also to the expensive cancer drugs [17,18]. The high cost of newer and targeted drug cancer is a significant obstacle to provision of medication for cancer patients in low and middleincome countries [19]. The Glivec* (imatinib) International Patient Assistance Program (GIPAP) is one of the approach strategies that work on reducing the burden of cancer in low and middle income countries.
The GIPAP in conjunction with the Max foundation and other Nongovernmental organizations has implemented a partnership with institutions (mostly hospitals) in developing countries to assist patients with CML and GIST by providing imatinib at no cost. Consequently as in high income countries, imatinib is the first line treatment in 28 countries in Africa eligible for the program. In these countries, despite the free access to imatinib, major cytogenetic responses have been reported unexpectedly low [10-13]. In Congo only 17% of CML patients had a major cytogenetic response [13]. Adherence to imatinib is questioned.
Our study revealed poor adherence to imatinib whatever the method we used to measure it. The degree of imatinib adherence varies depending on the studies and the method applied for the same purpose. Santoreli et al. in Italy reported an adherence rate at 83% [20]. The adherence was measured on 63 patients on imatinib using a software calculation measure based on the ratio of the received daily dose over the prescribed daily dose [20]. A study from the United Kingdom examined the adherence of imatinib by a microelectronic system monitoring fixed to the medication bottle [21]. The median adherence rate was 86% [21]. Darkow et al. in the US using the electronic data dispensation on patients found that 75 % where adherent to imatinib [22]. Chen et al. in Thailand included in a study 119 patients on imatinib and found that the median MPR was 98.3%. However, the MPR was lower than 60% for 10.1% of the patients [6]. Kappor et al. in India measured the adherence by using the Morisky scale [23]. They found that 75% of the patients scored = 11, therefore and were classified as adherent [23]. Among them 22% of the respondents scored 13, which means a perfect adherence [23]. Finally Al- Dewik in Qatar, found a lower adherence: 69% by using also the Morisky scale of adherence rate with a mean MPR at 94% [7].
Non-adherence is not limited to imatinib therapy. In fact, nonadherence is the prerogative of chronic diseases. Per WHO, adherence in chronic disease medications is globally low and probably lower in low and middle income countries [24]. When we compare with the Anti Retroviral Therapy (ART) for HIV which just like the imatinib, is provided at no cost to patients in Africa, the ART adherence was initially low. In early 2000 the adherence rate was ranging from 20 to 49.2% [25,26]. Adherence as well in the oncology area has been improved by different interventions such as information campaigns, booster consultations or short sending messages [25,26]. Adherence is a complex and multifactorial issue for which prospective studies are needed in the Congo to identify non-adherent factors and then tailor interventions to increase the imatinib adherence. While adherence has a common definition, it is also contextual, cultural and countries specific. While low MPR average in our study calls for a very important gap and interruption of the treatment in terms of weeks or months, in Western countries non-adherence to imatinib refers to an average of three doses missed per month [22].
Poor medication adherence is linked to a long term therapy. Difficulty in maintaining long term adherence is supported by Noens et al. who noticed that the adherence rate based on a 12 months imatinib treatment was lower than 6 months one [4]. In our study, discontinuation of imatinib occurred after a three months treatment.
The present study has a few limitations. First, the MMAS questionnaire was led by non trained interviewers who may have influenced the patients ‘answers, which explained the gap existing between adherence observed through interviews and pills counts. Second, the MMAS was translated for the purpose of the study in local dialect. The disadvantage of this approach is that the translation might have varied depending on the interviewer. It might also have slightly changed the content of the questionnaire and led to bias. Third, the MPR gives us a virtual idea of the patient adherence. It provides information on the number of imatinib pills provided to the patient but does not account if the medication was taken as prescribed. Finally, because of discontinuation the PCD instead of the MPR was the better tool to measure the imatinib adherence indirectly.
Conclusion
This initial study carried out in the African region demonstrates that adherence to imatinib with CML patients is poor. It is clearly understood that additional prospective studies will confirm or refute the present finding. Meanwhile, interventions are necessary to increase the patient’s adherence. Physicians and nurses need to strengthen relationships with their patients and educate them.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
The authors are grateful to GIPAP program for donating imatinib to Congolese CML patients, to the Max foundation and Axios International for faciliting shipping and delivery to the Congo.
We are also grateful to Professor Guy Laurent for his mentorship and assistance on this project and Professor Ngole Jean Pierre who kindly reviewed the manuscript.
References
- Martin LR, Summer LW, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005; 1: 189-199.
- Heinrich MC, Griffith DJ, Druker BJ,Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000; 96: 925-932.
- Druker BJ, Talpaz M, Debra J, Resta DJ, Bin Peng, Buchdunger E, et al. Efficacity and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. NEJM. 2001; 344: 1031-2037.
- Noens L,Van Lierde MA, De Bock R , Verhoef G, Zachée P, Berneman Z. Prevalence, determinants, and outcomes of non adherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009; 113: 5401-5411.
- Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, et al. Poor is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia on long term therapy. Blood. 2011; 117: 3733-3736.
- Chen TC, Chen LC, Huang YB, Chang CS. Imatinib adherence associated clinical outcomes of chronic myeloid leukemia in Taiwan. Int J Clin Pharm. 2014; 36: 172-181.
- Al-Dewik NI, Morsi HM, Samara MM, Ghasoub RS, Gnanam CC, Bhaskaran SK. Is adherence to imatinib mesylat treatment among patients better with CML associated with better clinical outcomes in Qatar? Clin Med Insights Oncol. 2016: 10: 95-104.
- Jonsson S, Olsson B, J. Soderberg, Wadenvik H. Good adherence to imatinib therapy among patients with chronic myeloid leukemia-a single center observational study. Ann Hematol. 2012; 91: 679-685.
- Wu EQ, Johnson S, Beaulieu N, Arana M, Bollu V, Guo A, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr.Med.Res.Opin. 2010; 26: 61-69.
- Durosinmi MA, Faloyi JO, Oyekule AA, Salawu L, Adediran IA, Akinola NO, et al. The use of imatinib mesylate in Nigerians with chronic myeloid leukemia. Cellular Therapy and transplantation. 2008; 1: 58-62.
- Segbena AY, Kueviakoe IMD, Agbetiafa K, Paradoro E, Layibo Y, Dorkenoo A, et al. Leucémie myéloïde chronique et imatinib. Expérience du CHU campus de Lomé au Togo. Médecine Santé et Tropical. 2010; 22: 307-311.
- Koffi KG, Nahno DC, N’dathz E, Kouehion P, Dissieka R, Attia A, et al. The effect of imatinib mesylate for newly diagnosed Philadelphia chromosomepositive, chronic phase myeloid leukemia in Sub-Saharan African patients: the experience of Côte d’Ivoire. Advances of Hematology. 2010: 268921:1- 10.
- Ngolet LO, Kocko I, Galiba Atipo Tsiba FO, Guelango Okuango Ova J, Elira Dokekias A et al. Imatinib mesylate in chronic myelogenous leukemia: A Congolese experience. EAMJ. 2016; 93: 118-122.
- Morisky DE, Ang A, Krousel-Wood M, Whard HJ. Predictive validy of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008: 10: 348-354.
- Trivedi D, Landsman-Blumberg P, Darkow T, Smith D, McMorrow D, Mullins CD. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J Manag Care Spec Pharm. 2014; 20:1006-1015.
- Giorfa S, Hofmann V, Bombaci F, Daban M, Efficace F, Guilhot J. Non adherence in chronic myeloid leukemia: result of a global survey of 2546 CML patients in 79 countries. 2014.
- Kanavos P. The rising burden of cancer in the developing world. Ann Oncol. 2006; 17: 15-23.
- Orem J, Wabinga H. The roles of national cancer research institutions in evolving a comprehensive cancer program in a developing country: experience from Uganda. Oncology. 2009; 77: 272-280.
- Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010; 376: 1186-1193.
- Santoleri F, Sorice P, Lasala R, Rizzo RC, Costantini A. Patient adherence and persistence with imatinib, nilotinib, dasatinib in clinical practice. Plos ONE. 2013; 8: 56813.
- Marin D, Bazeos A, Mahon FX , et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010; 28: 2361-88.
- Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs. A retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007; 25: 481-496.
- Kapoor J, Agrawal N, Ahmed R, Sharma SK, Gupta A, Bhurani D. Factors influencing adherence to imatinib in Indian chronic myeloid leukemia patients: a cross sectional study. Mediterr J Hematol Infec Dis. 2015; 7: 1-18.
- Sabaté E. Adherence to long term therapies: evidence for action. Geneva Switzerland: world Health Organization. 2003.
- Nwauche CA, Erhabor O, Ejele OA, Akani CI. Adherence to antiretroviral therapy among HIV infected subjects in a resource limited setting in the Niger Delta of Nigeria. Afr J Health Sci. 2006; 13: 13-17.
- Bangsberg RD, Perry S, Charlebois E, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral theray predicts progression to AIDS. AIDS. 2001; 15:1181-1183.
