
Research Article
Austin Neurol. 2021; 4(1): 1016.
Popular Ethnomedicinal Plant Alstonia scholaris Induces Neurotoxicity-Related Behavioural Changes in Swiss Albino Mice
Laskar YB, Laskar IH, Gulzar ABM, Vandana UK, Bhattacharjee N, Mazumder PB* and Bawari M
Department of Biotechnology, Natural Product & Biomedicine Research Laboratory, Assam University, Silchar, India
*Corresponding author: Pranab Behari Mazumder, Department of Biotechnology, Assam University, Silchar, India
Received: April 30, 2021; Accepted: May 27, 2021; Published: June 03, 2021
Abstract
Plants constituents are a reliable source of the remedial need of humanity for ages by being the basis of the traditional medicinal system and often serving as the prototype for designing modern medicine. Several plants are used in traditional medicine for ages without proper administration guidelines in terms of dosages. Several toxicological analyses revealed side-effects of such therapies beyond a specific dose. One such plant is Alstonia scholaris, widely used in numerous traditional medicines to treat diseases like ulcers, asthma, diabetes, etc. The present study investigated the neurotoxic effect of the plant extract through oxidative stress in Swiss albino mice. The treated mice showed anxiety, neophobic and depression-like properties compared to control mice. The biochemical parameters show an increase in Malondialdehyde (MDA) concentration while decreasing the total protein content in different brain regions of treated mice. The Glutathione Reductase (GR) activity shows an increase in treated mice compared to the control one. The study indicates that Alstonia scholaris may cause severe damage to the central nervous system when administered without a proper guideline.
Keywords: Alstonia scholaris; Neurotoxicity; In vivo; Malondialdehyde; Glutathione Reductase
Introduction
Traditional medicines that primarily include plant-based preparations are the most commonly used source of inexpensive and accessible remedies in many developing countries [1]. In developed countries, they are used as alternative medicines either with current therapies for synergistic effect or as a substitute for expensive treatments [2]. The low therapeutic index of many modern drug formulations also aided traditional medicines’ popularization in treating chronic illnesses [3]. Several clinically successful plantbased compounds and their derivatives are administered directly as alternative medicine or in combinational therapies to treat lifethreatening diseases like Cancer, Diabetes, Alzheimer’s, etc [4,5]. These compounds include taxols (Larotaxel, Paclitaxel, Cabazitaxel) for cancer treatment [6,7], Arteether/Artemotil (an artemisinin derivative) as anti-malarial [8], Galantamine for Alzheimer’s-related dementia [9], Apomorphine (a morphine derivative) for Parkinson’s disease [10], and Tiotropium (an atropine derivative) for pulmonary diseases [11]. The toxicological limitations of such clinically administered plant-based drug formulations are well-documented and followed extensively. However, no specific toxicological guideline is followed for plant-based preparations in traditional therapies that might have serious consequence [12-14]. Some recent studies proposed that several extensively used herbal formulations showed nephrotoxicity, neurotoxicity, cardiotoxicity, hepatotoxicity and reproduction-related side effects when used without a proper guideline of administration [15-18].
Alstonia scholaris, or the devil tree, belonging to the plant family Apocynaceae is a tropical tree commonly found in tropical South- East Asia, including India, China, Bangladesh up to the African and Australian continent [19]. In Indian Traditional Medicinal Systems (Ayurveda, Siddha, and Unani), the plant’s parts are used extensively to treat fever, chronic diarrhoea, dysentery, gout, rheumatism, skin diseases, malaria, jaundice, cancer, etc [19,20]. In Chinese traditional medicine, the plant’s parts are used to treat respiratory diseases, such as cough, asthma, phlegm, and COPD [19]. Additionally, its preparations are widely used in several countries and among several ethnic groups as a cost-effective alternative treatment for antipyretics in malaria, rheumatic pains, diabetes, and placenta expel after childbirth [19]. Although the plant’s preparations are used widely in traditional therapies, some reports suggest that the plant’s extracts may have several acute and chronic toxicity, including oral toxicity [21] and developmental toxicity [22] when used beyond a specific limit. Besides, Baliga et al. (2004) reported that high doses of Alstonia scholaris cause severe damage to all major organs of experimental rodent models [23]. The present study preliminarily evaluates the neurotoxic effect of the methanolic extract of the plant and the corresponding behavioural changes in experimental animals. The specific findings might help consider the neurotoxic side-effects while using the plant’s preparations in traditional therapies and regularizing standard administration doses.
Methodology
Preparation of plant extract
The leaf samples were collected from different locations of the Cachar district of Assam, India. The samples were dried under shade for seven days. The plant materials were extracted by following the methods described by Bello et al. (2016) [24] with a few modifications. Briefly, the dried samples were ground to powder and loaded in a cellulose thimble for extraction using a soxhlet apparatus. The repetitive extraction was carried out at 40°C using methanol as a solvent until the colourless solvent was observed. The collected extract was filtered through Whatman filter paper (No. 1). Further, the filtrate was concentrated by removing the solvent using a vacuum rotary evaporator. The extracted materials were kept in a -20°C refrigerator for further experiments. Later, two doses of the extract (100 and 300 mg/ml) were prepared as a low and high dose by dissolving them in distilled water for oral administration.
Experimental animals
Twenty-one (21) Swiss albino mice, about 8-10 weeks old (weighing between 25 and 30g), were obtained from the College of Veterinary Sciences at Assam Agriculture University, Guwahati, India. The animals were acclimatized to standard laboratory conditions for seven days before commencing the experiments. They were housed in large, clean polypropylene cages in a temperaturecontrolled room with 12hrs light and dark cycle, free access to water ad libitum and fed with a standard diet. Approval was obtained from the Institutional Animals Ethics Committee of Assam University, and all the ethical guidelines were followed strictly. After acclimatization, the animals were divided into three groups (n=7): Group A (served as standard control, administered with distilled water for seven days.), Group B (administered with ASME low dose) and Group C (administered with ASME high dose). The two concentration of the extract dissolved in distilled water were administered via oral gavage. The experimental animals were kept under regular and individual observation for body weight changes, behavioural changes and toxicity signs for the next seven days. Upon completing the experiments, the mice were sacrificed, and the blood/tissue samples were collected for biochemical assays.
Behavioural studies
Elevated plus maze test: The Elevated Plus Maze (EPM) Method is a regularly used behavioural assay for rodents that helps to estimate the anti-anxiety effects of pharmacological agents and steroid hormones [25]. The method has been employed in several recent impactful reports [26-28] that reflects the efficiency of the technique in accessing neurotoxicity-induced anxiety-related behaviour in rodents. The experiment was performed following the protocol described by Casarrubea et al. (2013) [29]. Briefly, the animals were transferred from the animal house to the experiment room in homecages to minimize the transfer effect. They were acclimatized for another 30 minutes to overcome any possible visual and olfactory influences. The EPM apparatus consisted of two open arms (16cm × 5cm) and two covered arms (16×5×12 cm). The arms extended from a central platform (5cm × 5cm), and the maze was elevated to a height of 25cm from the floor. Each animal was placed in the central platform, facing an open arm, and allowed to explore for 5min freely. The EPM apparatus was cleaned with ethyl alcohol (10%) after each observation to remove scent cues left from the preceding animal. The time spent by animals in the open arms, in the central platform and in the closed arms was calculated minute-by-minute and recorded for further analysis.
Modified hole board test: The Modified Hole Board Method (mHB) is another powerful method widely accepted to measure multiple dimensions of unconditioned behaviour in rodents [30]. Recently, many researchers used this method to validate their findings on the neurotoxic effect of different plant infusions that alters the locomotion and exploratory behaviour of experimental animals [31-33]. The grey wooden mHB box (60×60×25 cm) with sixteen equidistant holes (3cm in diameter) on the floor was raised 28 cm above the ground on a stand. Each equidistant hole was 28cm from the nearest wall of the box. The box was divided into squares of (20cm × 20cm). This experiment was performed by following the protocols described by Pyrzanowska et al. (2021) [33] with few modifications. Briefly, the experimental animal was placed in the corner facing the wall and allowed to explore for 5 minutes freely. The total number of heads-dips recorded. A mouse was considered to make a head-dip if both eyes disappeared into the hole. The box was cleaned with 10% alcohol after each observation to avoid any bias based on olfactory cues.
Forced swim test: The Forced Swim Test (FST) is a rodent behavioural test used to evaluate the depression-like symptoms and stress-coping behaviour in experimental animals when treated with new compounds/drugs or neurotoxic agents [34-36]. The FST was performed according to the method described by Tanaka et al. (2020) [37] recently. Briefly, the individual mice were placed in a cylindrical glass container of 12 cm in diameter and 30 cm in height and left there for 5min. The cylinder was filled with water (25±1 °C) to a height of 20cm. The duration of swimming and immobility was recorded. Freshwater was used for each mice. A mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water [38].
Biochemical assays
Following the behavioural studies, the mice were sacrificed immediately, and the brain tissues (cerebral cortex) were isolated by dissection. The tissues were washed with ice-cold saline, blot dry with filter paper, weighed and homogenized in a cool environment. The homogenates were used for the biochemical analyses.
Lipid peroxidation (LPO) assay: Lipid peroxidation by Reactive Oxygen Species (ROS) is a well-established detrimental mechanism for several acute and chronic brain disorders [39]. The Thiobarbituric Acid assay (TBA test) developed by Ohkawa et al. (1979) [40] is still used as a robust biochemical assay for detecting lipid peroxides in animal tissues. Thiobarbituric Acid (TBA) reacted with Malondialdehyde (MDA), which resulted in a colour compound. Briefly, to 0.2ml of the test sample, 0.2ml of SDS, 1.5ml of acetic acid and 1.5ml TBA was added. The mixture was made up to 4ml with water and then heated in a water bath at 95°C for 60 minutes. After cooling, 1ml of water and 5ml of n-butanol/pyridine mixture was added and shaken vigorously. After centrifuging at 4000 rpm for 10 minutes, the organic layer was formed, and its absorbance was measured spectrophotometrically at 532nm. The level of lipid peroxidation was expressed as nmoles of MDA released per gram of wet tissue. The concentration of MDA was expressed using the formula: Absorbance at 532nm × D /L × €, where L= light path (1cm), D=Dilution factor and, €= extinction coefficient (1.56×105M-1cm-1).
Total protein estimation: The total protein content of the samples was assessed using Folin-Lowry’s method of protein estimation [41]. The absorbance of standards was measured at 550nm against the blank, and a standard curve was generated. From this standard curve, the protein concentration for samples was calculated.
Glutathione reductase (GR) activity assay: The GR activity in the experimental tissue samples was determined spectrophotometrically at 340nm by following Carlberg and Mannervik (1975) method [42]. Briefly, the GR activity was determined by measuring the oxidation of NADPH at 340nm in a reaction mixture containing 1.8ml phosphate buffer, 300μl each of EDTA, NADPH, oxidized glutathione (GSSG) and enzyme extract. The unit of GR activity was expressed as the amount that catalyzes the consumption of 1μmol of substrate per minute, using a molar extinction coefficient of 6.2mM-1cm-1 for NADH/NADPH.
Results
Change in body weights
Body weights of both the control and treated animals were measured on an alternative day until the experiments. The body weights of LD-treated and HD-treated animals showed a 2.58% and 0.23% increased in weight, respectively. In contrast, control animals showed a 5.18% weight gain on the 7th-day than the initial body weight (Figure 1).
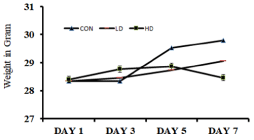
Figure 1: Effect of Alstonia scholaris Low-dose (LD) and High-dose (HD) on
the body weights of the treated mice in comparison to the control (CON) mice.
Elevated plus maze test
EPM test was performed to understand the variation of anxiety that occurred in different treated groups. The anxiety is estimated by the difference between the time spent in the open arm and the closed arm. More time spent in the closed arm indicates more anxiety, i.e. anxiogenic mice. The observations were cross- validated by treating the mice with diazepam, a popular anxiolytic drug. Diazepam treatment reduced the anxiety symptoms in ASME-treated mice as the mice marked more occurrence in the open arms of the EPM apparatus. On day one, no significant change in anxietyrelated behaviour was observed in any group after ASME treatment. However, after extended exposure, the treated mice showed an increase in anxiogenic behaviour than the control. On day four, the LD-treated and HD-treated mice showed a 1.3 and 1.29 fold increase in anxiogenic behaviour, respectively. On day seven, the anxiogenic behaviour in HD-treated mice increased significantly by 1.19 fold, whereas the LD-treated group showed only a 1.03 fold surge. The time spent (in seconds) by different groups in the closed arm and open arm of the EPM apparatus is shown in Figure 2 and Figure 3. These observations indicate that ASME could induce anxiety in rodents in a dose and duration-dependent manner.
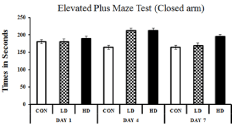
Figure 2: Showing the effect of ASME treatment on the anxiogenic behaviour
of experimental animals: The time spent (in seconds) by different groups in
the open arm of the EPM apparatus.
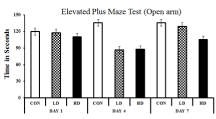
Figure 3: Showing the effect of ASME treatment on the anxiogenic behaviour
of experimental animals: The time spent (in seconds) by different groups in
the open arm of the EPM apparatus.
Modified hole board test
The Modified Hole Board (mHB) test is a measure of exploratory (neophilia/neophobic) behaviour. The Exploratory Behaviour (EB) was measured by the number of head-dips made by the animals into the hole when kept in a new/unknown environment. The EB of the animal varied marginally on the 1st day of ASME exposure. However, on prolonged exposure, the mice showed signs of anxiety and reduced EB. On day four, the EB of LD-treated and HD-treated mice marked a 23.16% and 34.74% fall from control, respectively. The anxiety-induced decrease in EB was higher on the 7th day as well, and the mice showed neophobic activity with 31.31% (LD-treated) and 36.36% (HD-treated) reduced exploratory activity compared to the control (Figure 4). However, diazepam treatment improved the exploratory behaviour in the experimental groups, indicating that anxiety caused the neophobic signs in those mice.
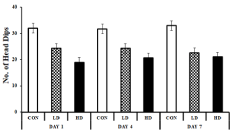
Figure 4: Showing the effect of ASME treatment on the exploratory behaviour
of experimental animals: The Y- axis ressembels the no. of head-dips in
5minutes on a mHB test.
Forced swim test
The Forced Swim Test (FST) was performed to explore the effect of ASME treatment on inducing depression-like symptoms on experimental animals. An increased immobility time in FST indicates depression signs in rodent models [43]. The test was performed on the 7th day of the experimental setup before sacrificing the mice. The LDtreated and HD-treated mice exhibited a respective 1.85 and 2.06 fold increase in immobility and an increased floating time compared to the control group (Figure 5). Moreover, normal swimming tendency was observed in the experimental animal on treating with fluoxetine, an approved antidepressant. These observations indicate that ASME could induce depression-like symptoms in mice in a dose-dependent manner.
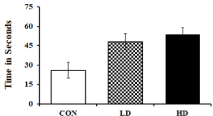
Figure 5: ASME treatment induced depression-like symptoms on
experimental animals: The figure indicating the immobility time of animal in
FST after treatment with low-dose and high-dose of ASME.
Lipid Peroxidation (LPO) assay
The lipid peroxidation was estimated spectrophotometrically by measuring the concentration of Malondialdehyde (MDA) formed in the tissue samples. A higher value of MDA indicates elevated lipid peroxidation that leads to several chronic and acute brain ailments [44,45]. The MDA concentrations in the mice cortex tissue homogenates of the control, LD-treated and HD-treated groups were found to be 37±1.53, 51.47±6.26 and 53.67±1.86 μM/g, respectively (Figure 6). The MDA concentration elevated in the LD- treated and HD-treated groups by 1.39 and 1.45 fold, respectively, compared to the control. MDA concentrations were also found to be elevated in the cerebellum tissues by 1.46 and 1.53 fold than the control. The LD and HD-treated groups exhibited 49±7.37 and 51.6±3.95 μM/g MDA, where control groups showed only 33.67±0.88 μM MDA per gram of tissue homogenate (Figure 6). The results indicate that ASME treatment significantly increases the lipid peroxidation in the mice brain tissues that may have resulted in anxiety and depression like symptoms in the treated groups.
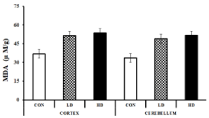
Figure 6: Effect of ASME on Malondialdehyde (MDA) level in the brain of
Swiss albino mice.
Total protein estimation
The total protein content of different tissues also showed a subsequent decline from the control to the higher doses. The protein content of the cortex tissues for the control, LD and HD-treated groups were found to be 113.57±4.46, 110±1.89 and 73.57±15.67 mg/g, respectively. Although the low dose of ASME declined the protein content by only 3.14% compared to the control, a high dose reduced them significantly by 35.22%. In cerebellum tissues, the total protein content was reduced by 27.42% to 64.29±6.19 mg/g by the low dose of ASME and 51.61% to 42.86±3.27 mg/g by the high dose as compared to the control (88.57±1.89 mg/g). Thus, ASME treatment dramatically reduced the total protein content of the brain in a dosedependent manner.
Glutathione reductase (GR) activity assay
Glutathione Reductase (GR) is an antioxidant enzyme that plays a vital role in regulating cellular redox homoeostasis by controlling the cellular level of ROS [46]. Usually, the cellular GR level increases as a response to stress induced by ROS-generating agents [47]. The GR levels in the mice cortex tissues were found to be 0.038±0.002, 0.043±0.001 and 0.052±0.004 μM/min/mg protein, respectively for the control group, LD and HD-treated group. The values for the LD and HD-treated groups were raised by 1.13 and 1.37 fold compared to the control. The cerebellum tissues also showed similar trends of elevated GR levels with the values 0.051±0.001, 0.059±0.005 and 0.068±0.004 μM/min/mg protein from lower dose to the higher dose groups (Figure 8). The elevated GR level might be a response to the ROS generated by ASME treatment.
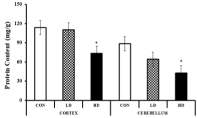
Figure 7: Effect of ASME on the total protein content of the brain of Swiss
albino mice.
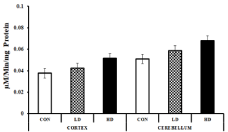
Figure 8: Effect of ASME treatment on the glutathione reductase activity in
the cortex and cerebellum tissues of Swiss albino mice.
Discussion
Traditional and complementary medicines are widely used globally, especially in India and China. The Indian Traditional Medicinal system of Ayurveda and Chinese Traditional Medicine (CTM) primarily uses plant-based preparations for treating almost every disease. Because plant-based therapies are mainly used as food or supplement, their use is not adequately regulated as the modern synthetic drugs. Ethnomedicinal research primarily reports the pharmacological activity of plants based on their ethnic or historical use but lack proper toxicological analysis of those plant extracts. The Toxicological Risk Assessment (TRA) of any experimental chemical or drug mainly includes evaluating its toxic effect on the major organs like the brain, heart, kidneys and liver [48]. The toxicity risk associated with the Central Nervous System (CNS), known as neurotoxicity, is a significant step in the TRA process that determines the adverse effect of any chemical/toxin/drug on the normal functioning of the CNS [49]. Also, it is essential to determine the neurotoxic effect of a chemical because the CNS regulates many other body systems like the digestive system, the circulatory system and the immune system in a very complex way [49]. It is found that several herbal medicines possess neurotoxins resulting from misidentification of the parent plant, insufficient processing, or adulteration [16]. When administered without a proper TRA and guideline for doses, these formulations can cause severe damage to the functioning of the CNS.
In the present study, some behavioural and biochemical parameters were observed in Swiss albino mice after treating them with 100mg/ml (LD) and 300mg/ml (HD) of Alstonia scholaris methanolic extract. The treated mice showed some fluctuation in their body weight, where the HD-treated mice showed significant weight loss compared with the control group. This significant body weight loss indicates an adverse effect of the high dose of ASME [50]. The doses also demonstrated significant behavioural changes in the treated mice. The observation in the EPM is widely used for screening potential anxiolytics and their effect on the exploration, anxiety, and motor behaviour of rodents [51]. The anxiogenic rodent tries to avoid the open arms of the maze and hides in the arms enclosed by protective walls [51]. Both the experimental groups (LD and HD-treated) showed similar anxiogenic behaviour, which was then cured by treating with diazepam. Since diazepam acts by enhancing the calming effect of Gamma-Aminobutyric Acid (GABA) by facilitating the opening of the GABAA-activated chloride channels, it can hypothesize that ASME constituents block the action of GABA. Similar anxiety and depression-like signs were displayed by both the treated group in mHB and FST. Although the degrees of behavioural changes were consistently higher in the HD-treated group, suggesting that the neurotoxic effect of ASME is dose-dependent. The recovery of behavioural changes to normalcy after treatment with anxiolytic drug diazepam in all test cases confirms the onset of anxiety and depressionlike symptoms induced by ASME doses. Similar anxiogenic results were observed by Elsas et al. (2010) [52] and Sonavane et al. (2002) [53] in male Sprague-Dawley albino rats treated with the extracts of Passiflora incarnata L and Myristica fragrans, respectively.
The high levels of lipid peroxidation (measured by malondialdehyde levels) and glutathione reductase in the cortex and cerebellum region of the brain indicate that ASME induces neurotoxicity through ROS- generation. The harmful reactive oxygen species or ROS are constantly generated through normal oxidative metabolism or incomplete metabolism of foreign chemicals by the liver. ROS play a central role in the onset of several neurodegenerative diseases by causing progressive loss of neurons [54]. Lipid peroxidation in the brain is a typical response to high ROS levels that severely damage the polyunsaturated fatty acids rich neuronal membranes [55]. Malondialdehyde (MDA) is the most abundant lipid peroxidation-specific aldehyde and is highly reactive with proteins, DNA, and phospholipids, causing harmful effects [55]. The Thiobarbituric Acid (TBA) assay is performed widely to measure the red pigmentation formed by the adduct of TBA with MDA [55]. The high MDA levels estimated in the present study demonstrates oxidative stress- induced lipid peroxidation in the mice brain tissues. Consequently, high levels of antioxidant enzyme Glutathione Reductase (GR) were also recorded in the mice brain tissues, a detoxification mechanism against oxidative stress [56]. The study results also demonstrated that ASME extracts altered neuroprotein levels in the mouse cerebral cortex and the cerebellum, indicating that prolonged high doses may disturb neurodevelopment.
Conclusion
In conclusion, the study found that Alstonia scholaris extract dose-dependently induces neurotoxicity, resulting in signs of anxiety and depression in Swiss albino mice. Further, high doses of the plant extract may increase lipid peroxidation and oxidative damage to mice brain. The information obtained from this study may help to choose more appropriate test doses of Alstonia scholaris in future pharmacological studies to report results of greater clinical relevance. Lastly, caution and safety measures must be followed while using Alstonia scholaris for therapeutic or nutraceutical purposes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for-profit sectors.
Acknowledgement
We want to acknowledge DBT-Electronic Library Consortium (DeLCON) for providing access to research articles and UGC-SAP for providing laboratory infrastructure. The first, second, third and fifth author gratefully acknowledges the UGC-AUS Non-NET fellowship received from University Grant Commission (India) through Assam University, Silchar, towards doctoral research.
References
- O Oyebode, N-B Kandala, PJ Chilton and RJ Lilford. “Use of traditional medicine in middle- income countries: a WHO-SAGE study”. Health Policy Plan. 2016; 31: 984-991.
- World Health Organization. “WHO Global Report on Traditional and Complementary Medicine 2019”. 2019.
- H Wen, H Jung and X Li. “Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges”. AAPS J. 2015; 17: 1327-1340.
- A SAKLANI and S KUTTY. “Plant-derived compounds in clinical trials”. Drug Discov. Today. 2008; 13: 161-171.
- O Potterat and M Hamburger. “Drug discovery and development with plantderived compounds”. In Natural Compounds as Drugs Volume I, Basel: Birkhäuser Basel. 45-118.
- MA Ashraf. “Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty”. Biomed Res. Int. 2020; 2020: 1-7.
- A Seca and D Pinto. “Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application”. Int. J. Mol. Sci. 2018; 19: 263.
- B Afolabi and CA Okoromah. “Intramuscular arteether for treating severe malaria”. Cochrane Database Syst. Rev. 2004.
- S Berkov, et al. “Plant Sources of Galanthamine: Phytochemical and Biotechnological Aspects”. Biotechnol. Biotechnol. Equip. 2009; 23: 1170- 1176.
- F Carbone, A Djamshidian, K Seppi, and W Poewe. “Apomorphine for Parkinson’s Disease: Efficacy and Safety of Current and New Formulations”. CNS Drugs. 2019; 33: 905-918.
- A Salim, Y-W Chin and AD Kinghorn. “Drug Discovery from Plants”. In Bioactive Molecules and Medicinal Plants, Berlin, Heidelberg: Springer Berlin Heidelberg. 2008: 1-24.
- Schilter, et al. “Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements”. Food Chem. Toxicol. 2003; 41: 1625-1649.
- WM Bandaranayake. “Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs”. In Modern Phytomedicine, I Ahmad, F Aqil and M Owais. Eds. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. 2006: 25-57.
- DH Phua, A Zosel and K Heard. “Dietary supplements and herbal medicine toxicities-when to anticipate them and how to manage them”. Int. J. Emerg. Med. 2009; 2: 69-76.
- B Yang, Y Xie, M Guo, MH Rosner, H Yang and C Ronco. “Nephrotoxicity and Chinese Herbal Medicine”. Clin. J. Am. Soc. Nephrol. 2018; 13: 1605-1611.
- EM Williamson. “Herbal Neurotoxicity: An Introduction to Its Occurrence and Causes”. In Toxicology of Herbal Products, Cham: Springer International Publishing. 2017: 345-362.
- P Zhang, Y Ye, X Yang and Y Jiao. “Systematic Review on Chinese Herbal Medicine Induced Liver Injury”. Evidence-Based Complement. Altern. Med. 2016; 2016: 1-15.
- E Stournaras. “Herbal medicine-related hepatotoxicity”. World J. Hepatol. 2015; 7: 2189.
- S Akbar. “Alstonia scholaris (L.) R.Br. (Apocynaceae)”. In Handbook of 200 Medicinal Plants, Cham: Springer International Publishing. 2020: 225-234.
- MS Khyade, DM Kasote and NP Vaikos. “Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: A comparative review on traditional uses, phytochemistry and pharmacology”. J. Ethnopharmacol. 2014; 153: 1-18.
- Bello, et al. “Acute and Sub-Acute Toxicity Evaluation of the Methanolic Extract of Alstonia scholaris Stem Bark”. Med. Sci. 2016; 4: 4.
- GC Jagetia and MS Baliga. “Induction of developmental toxicity in mice treated with Alstonia scholaris (Sapthaparna) In utero”. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003; 68: 472-478.
- M BALIGA. “The evaluation of the acute toxicity and long term safety of hydroalcoholic extract of Sapthaparna (Alstonia scholaris) in mice and rats”. Toxicol. Lett. 2004; 151: 317-326.
- Bello et al. “Acute and Sub-Acute Toxicity Evaluation of the Methanolic Extract of Alstonia scholaris Stem Bark”. Med. Sci. 2016; 4: 4.
- A Walf and CA Frye. “The use of the elevated plus maze as an assay of anxiety-related behavior in rodents”. Nat. Protoc. 2007; 2: 322-328.
- NB Bertagna, PGC dos Santos, RM Queiroz, GJD Fernandes, FC Cruz and TT Miguel. “Involvement of the ventral, but not dorsal, hippocampus in anxietylike behaviors in mice exposed to the elevated plus maze: participation of CRF1 receptor and PKA pathway”. Pharmacol. Reports. 2021; 73: 57-72.
- N Sotoudeh, MR Namavar, A Zarifkar and AR Heidarzadegan. “Agedependent changes in the medial prefrontal cortex and medial amygdala structure, and elevated plus-maze performance in the healthy male Wistar rats”. IBRO Reports. 2020; 9: 183-194.
- J Schrader, RM Taylor, EG Lowery-Gionta, and NLT Moore. “Repeated elevated plus maze trials as a measure for tracking within-subjects behavioral performance in rats (Rattus norvegicus)”. PLoS One. 2018; 13: e0207804.
- M Casarrubea et al. “Temporal structure of the rat’s behavior in elevated plus maze test”. Behav. Brain Res. 2013; 237: 290-299.
- M Labots, HA Van Lith, F Ohl, and SS Arndt. “The Modified Hole Board - Measuring Behavior, Cognition and Social Interaction in Mice and Rats”. J. Vis. Exp. 2015; 98.
- A Apu, F Hossain, S Bhuyan, F Rizwan, M Matin, and AT Jamaluddin. “Study of pharmacological activities of methanol extract of Jatropha gossypifolia fruits”. J. Basic Clin. Pharm. 2013; 4: 20.
- M Shahed-Al-Mahmud and SMM Lina. “Evaluation of sedative and anxiolytic activities of methanol extract of leaves of Persicaria hydropiper in mice”. Clin. Phytoscience. 2017; 3: 20.
- Pyrzanowska et al. “Aspalathus linearis infusion affects hole-board test behaviour and amino acid concentration in the brain”. Neurosci. Lett. 2021; 747: 135680.
- A Can, DT Dao, M Arad, CE Terrillion, SC Piantadosi, and TD Gould. “The Mouse Forced Swim Test”. J. Vis. Exp. 2011; 58.
- G Commons, AB Cholanians, JA Babb, and DG Ehlinger. “The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior”. ACS Chem. Neurosci. 2017; 8: 955-960.
- PJ Fitzgerald, JY Yen, and BO Watson. “Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test”. PLoS One. 2019; 14: e0215554.
- M Tanaka, Z Bohár, D Martos, G Telegdy and L Vécsei. “Antidepressant-like effects of kynurenic acid in a modified forced swim test”. Pharmacol. Reports. 2020; 72: 449-455.
- B Petit-Demouliere, F Chenu and M Bourin. “Forced swimming test in mice: a review of antidepressant activity,” Psychopharmacology (Berl). 2005; 177: 245-255.
- YJ Garcia, AJ Rodríguez-Malaver and N Peñaloza. “Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices”. J Neurosci. Methods. 2005; 144: 127-135.
- H Ohkawa, N Ohishi, and K Yagi. “Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction”. Anal. Biochem. 1979; 95: 351-358.
- OH LOWRY, NJ ROSEBROUGH, AL FARR and RJ RANDALL. “Protein measurement with the Folin phenol reagent”. J. Biol. Chem. 1951; 193: 265- 275.
- A Carlberg and B Mannervik. “Purification and characterization of the flavoenzyme glutathione reductase from rat liver”. J. Biol. Chem. 1975; 250: 5475-5480.
- V Castagné, P Moser, S Roux, and RD Porsolt. “Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice”. Curr. Protoc. Neurosci. 2011; 55.
- D Achitei, A Ciobica, G Balan, E Gologan, C Stanciu, and G Stefanescu. “Different Profile of Peripheral Antioxidant Enzymes and Lipid Peroxidation in Active and Non-active Inflammatory Bowel Disease Patients”. Dig. Dis. Sci. 2013; 58: 1244-1249.
- M Bilici, H Efe, MA Köroglu, HA Uydu, M Bekaroglu and O Deger. “Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments”. J. Affect. Disord. 2001; 64: 43-51.
- N Couto, J Wood, and J Barber. “The role of glutathione reductase and related enzymes on cellular redox homoeostasis network”. Free Radic. Biol. Med. 2016; 95: 27-42.
- A Bhattacharyya, R Chattopadhyay, S Mitra, and SE Crowe. “Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases”. Physiol. Rev. 2014; 94: 329-354.
- World Health Organization. “Principles and Methods for the Risk Assessment of Chemicals in Food (Public Health)”. World Health Organization. 2010: 3-190.
- S Spencer and PJ Lein. “Neurotoxicity”. In Encyclopedia of Toxicology. Elsevier. 2014: 489-500.
- A Tahraoui, ZH Israili and B Lyoussi. “Acute and sub-chronic toxicity of a lyophilised aqueous extract of Centaurium erythraea in rodents”. J. Ethnopharmacol. 2010; 132: 48-55.
- HS Foyet, DE Tsala, AA Bouba, and L Hritcu. “Anxiolytic and Antidepressant-Like Effects of the Aqueous Extract of Alafia multiflora Stem Barks in Rodents”. Adv. Pharmacol. Sci. 2012; 2012: 1-8.
- S-M. Elsas, et al. “Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method”. Phytomedicine. 2010; 17: 940-949.
- G Sonavane, V Sarveiya, V Kasture, and S Kasture. “Anxiogenic activity of Myristica fragrans seeds”. Pharmacol. Biochem. Behav. 2002; 71: 239-244.
- F Collin. “Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases”. Int. J. Mol. Sci. 2019; 20: 2407.
- M Shichiri. “The role of lipid peroxidation in neurological disorders”. J. Clin. Biochem. Nutr. 2014; 54: 151-160.
- MM Djukic, et al. “Protective role of glutathione reductase in paraquat induced neurotoxicity”. Chem. Biol. Interact. 2012; 199: 74-86.