
Research Articles
Austin J Autism & Relat Disabil. 2015; 1(2): 1009.
From a Single Gene Defect Towards a Cross Species Neurocognitive Phenotype: The EHMT1 Disruption Example (Kleefstra Syndrome)
Karlijn Vermeulen1,2,3*, Wouter G. Staal1,3*, Joost G. Janzing², Jan K. Buitelaar1,3, Hans van Bokhoven3,4,5, Jos IM Egger6,7,8 and Tjitske Kleefstra3,4
¹Karakter Child and Adolescent Psychiatry University Centre, Netherlands
²Department of Psychiatry, Radboud University Medical Centre, Netherlands
³Department of Cognitive Neuroscience, Donders Institute for Brain, Cognition and Behavior, Radboud University Medical Centre, Netherlands
4Department of Human Genetics, Radboud University Medical Centre, Netherlands
5Centre for Molecular Life Sciences, Radboud University Nijmegen, Netherlands
6Centre for Cognition, Donders Institute for Brain, Cognition and Behavior, Radboud University Nijmegen, Netherlands
7Vincent van Gogh Centre of Excellence for Neuropsychiatry, Netherlands
8Behavioral Science Institute, Radboud University Nijmegen, Netherlands
*Both are equally contributing to this work
*Corresponding author: Tjitske Kleefstra, Department of Human Genetics, Radboud University Nijmegen Medical Centre, P.O. Box 9101, 6500 HB Nijmegen, Netherlands
Received: March 07, 2015; Accepted: September 10, 2015; Published: September 25, 2015
Abstract
Aim: Neuropsychiatric disorders comprise a clinically heterogeneous group of conditions that share underlying molecular and pathological mechanisms. Knowledge about how molecular pathways affect cognition and emotion is highly fragmentary. The Euchromatin Histon Methyl Transferase 1 (EHMT1) gene encodes a protein involved in chromatin modification and is presumed to have an important role in the etiology of cognitive and emotional dysfunctions. While animal studies with EHMT mutant species showed deviations in learning, attention and social cognition, systematic studies investigating behavioral characteristics in humans have been hardly conducted. Adult case studies showed behavioral disturbances with a sudden decline in functioning together with sleep problems. The focus of this report is to describe symptoms and developmental aspects in children with EHMT1 defects and compare these two previous studies in adults and animal models.
Methods: Four children with EHMT1 defects were investigated on adaptive functioning, psychiatric symptoms and temperament.
Results: All subjects have a severe developmental delay and meet the criteria for autism spectrum disorders, irrespective of severity of their intellectual disability. Three subjects have extreme sleep problems, while two subjects also have anxiety and mood disorders. Temperament is characterized by deficits in attentional focusing and inhibition.
Conclusion: These results show that EHMT1 defects severely affect cognitive processing, behavior and emotional functioning in children. This is in agreement with previous observations in animal studies and adult patients. The important role of EHMT1 in cognitive processing suggests that disruptions in a single gene can result in a broad spectrum of psychiatric pathology in human.
Keywords: Kleefstra syndrome; EHMT1 gen; Intellectual disability; Autism; Sleep; Behavior
Introduction
Twin, adoption and molecular genetic studies have produced evidence that genetic factors are significant contributors to the etiology of several neuropsychiatric disorders and related traits. Although the genetic architecture of these disorders is complex, it has become evident that rare, moderate risk variants such as de novo Copy Number Variants (CNVs) are an important contributor to the disease risk [1-5]. Identification of harmful CNVs may be challenging, but even more difficult is defining how such genomic changes affect biological pathways and result in disease.
Known disruptions in a specific gene, well described in terms of biological functioning and associated phenotype will be of vital importance to understand the role of CNVs in neuropsychiatric disorders. Disruptions of EHMT1 are a clear example of a specific genetic abnormality that results in psychiatric symptoms. In a recent study using Next Generation Sequencing in humans with various cognitive dysfunctions, EHMT1 was identified as the only common mutated gene in an overlapping group of cognitive disorders: Intellectual Disability (ID), autism and schizophrenia [6]. The EHMT1 gene is located on 9q34 at the subtelomeric end. Either 9q34 micro deletions or intragenic EHMT1 mutations can similarly lead to impaired cognitive functioning [7]. The gene is expressed widely in neural tissue strongly suggesting that the EHMT1 gene plays an important role in neurodevelopment. The enzyme, for which it encodes, is capable to methylate lysine 9 of histone 3 (K9H3 methylase). Consequently, changes in chromatin modulation occur, thereby influencing epigenetic processes of gene regulation [8,9]
Importantly, the EHMT gene is studied cross species. Behavioral studies in Drosophila melanogaster and mice with EHMT defects have been performed [10,11] and show strong associations between EHMT defects and neurocognitive abnormalities. For example, in Drosophila, neurodevelopment and behavioral analyses identified EHMT as a regulator of larval loco motor behavior, non-associative learning, and courtship (complex) memory. Moreover, memory dysfunction was repaired by EHMT re-expression during adulthood, indicating that cognitive defects are reversible in EHMT Drosophila mutants [11]. Additionally, the study of heterozygous EHMT1 knockout mice demonstrated difficulties in the processing of novel stimuli, higher levels of anxiety and a deviation in social reaction to strangers [10].
In humans, mutations of EHMT1 result in a clinical syndrome, known as Kleefstra Syndrome (KS) or chromosome 9q sub telomere deletion syndrome [OMIM 610253]. Worldwide about 150 cases of this syndrome are reported so far [12-14]. The clinical picture of this syndrome is dominated by intellectual disability, hypotonia and characteristic facial dysmorphisms such as micro-/brachycephaly, mid-face hypoplasia, hypertelorism, synophrys with/without arched eyebrows, a short nose and tongue-protrusion. These can be accompanied by a variety of symptoms as congenital structural heart defects, uro-genital defects, epilepsy and severe behavioral disturbances.
Two recent reports pay attention to the behavioral characteristics in a few adults with the KS [15,16]. These patients showed clinical features like apathy and severe sleep problems and significant loss of functioning became apparent after adolescence with increasing symptoms over time. This “regressive” phenotype was suggested to be associated with the EHMT1 gene particularly since it was also observed in a patient with an intragenic EHMT1 mutation [16].
To date, no studies are reported that specifically and systematically investigate symptoms and behavioral characteristics of children with KS. Consequently, it is as yet unknown which symptoms do occur due to the EHMT1 mutation and to what degree.
Therefore, the present study focuses on mapping symptoms and developmental aspects in childhood and compares these to the results in animal studies and in adult patients, thereby connecting a single gene disruption with a biological pathway and with behavior. In addition, this cross species comparison may be of help in future studies that address development of therapeutic potentials of affected chromatin modification.
Materials and Methods
Participants
In this pilot study, four subjects with an EHMT1 defect participated: Three males etc., and one female. The participants were referred by the department of Human Genetics, Radboud University Medical Center Nijmegen, Netherlands. Informed consent was obtained from legal representatives (parents), because the subjects aren’t competent to sign for participation. However, if a subject indicated not wanting to participate or wanting to quit a task, then this was immediately followed by termination of the task or participation. The informed consent was signed by the parents on a consent form, which is included in the file of the participant. The regional medical ethics committee (Medical Ethics Committee of the Radboud University Medical Centre, Nijmegen, Netherlands) approved the consent procedure. Their ethics statement is positive about the investigation file.
Patient characteristics
Patient characteristics and developmental aspects are listed in table 1. Detailed descriptions of the subjects are added in the appendix. The medical and genetic characteristics of all cases have been published in short in previous clinical genetic studies; patient 1 was reported as patient 21 in [14] . Patient 2 and patient 3 were reported as patient 4 and patient 8 respectively in [7, 14], patient 4 was reported as patient 27 in [14]. Patient 1, 2 and 4 had an intragenic EHMT1 mutation; patient 3 had a 9q34.3 micro deletion encompassing entire EHMT1 and other genes (3.2 Mb).
Patient 1
Patient 2
Patient 3
Patient 4
Age/ gender
Genetic defect
5 years, male
EHMT1 mutation
c.2785_2788del
(p.Ser929fs)10, male
EHMT1 mutation
c.3409C>T
(p.R1137*)10, male
EHMT1 deletion
3,2 Mb (137,04-140,21 MB, UCSC, NCBI build 36.1)11, female
EHMT1 mutation
c.3072_3073del
(p.Val1026fs)Developmental characteristics
Birth:Gestation (weeks + days)
Birth weight
Abnormalities
33+4
1750 g
Breathing problems
Incubator (1 month)
41
3480 g
Meconium in amniotic fluid
32
2950 g
Blood loss and minimal fetal movements during pregnancy.
Omphalocele and marked hypotonia at birth. After birth: incubator, feeding by fluid infusion
41
3600 g
Induced labor with vacuum extraction
Respiratory problems at birth
Infancy
Floppy baby
Feeding difficulties
Floppy baby
Feeding difficulties
Poor contact with parents
Floppy baby
Feeding difficulties
Overstretching after feeding
Poor contact with parents
Easy to comfort
Feeding difficulties after switching to solid food.
Toddlerhood:
Milestones (year; months)
Laugh
Walk
TalkAbnormalities
Delayed
0;8
4;3
2;6
Special interest in light, moving parts of objects, animals and music.
Delayed
1;0
9;0
Not able to talk
Special interest in a specific toy with a sound
Delayed
0;3
5;0
Not able to talk
Fascination with technical aspects, water and music
Delayed
0;2
2;0
5;0
Special interest in music and water.Somatical Highlights
Recurrent airway infections
Tantrums
Absences (decreased without treatment)
Dullness of hearing
Reflux in infancy
Recurrent infections: airway, bladder.
Regurgitation in infancy
Hearing and visual difficulties.
Extreme obstipation together with mood swings at 10 years
Medication
Current
Past
Risperidone Valproaat
None
Pipamperon, Omeprazol
Cotrimoxazol (maintainance treatment)Olanzapine, Valproate
Macrogol, Bisacodyl
Table 1: Patient Characteristics.
Cognitive and behavioral measures
(Mal) Adaptive functioning. VABS. the dutch adaptation of the vineland adaptive behavior scale (VABS); [17]: This is a widely used clinical interview to determine the level of adaptive functioning of people with an intellectual disability. The VABS consists of 3 domains: communication skills, daily living skills and social skills. This instrument has a good reliability and validity in this specific population [18]. Primary caregivers were interviewed about the participants.
The mini psychiatric assessment schedules for adults with developmental disabilities (mini PAS-ADD); [19]; in dutch translation [20]: This is used to determine behavioral problems and psychiatric disease in subjects with an intellectual disability by interviewing the proxy. It consists of 86 items on a 4-point scale: 0 (symptom not present) -3 (symptom is severe). The interview is divided into seven subscales: Depression, Anxiety, Obsessive/ Compulsive disorder, Hypomania/Mania, Psychosis, Unspecified disorder and Autism. All criteria are based on the International Classification of Diseases (ICD-10). This instrument has proven psychometric qualities in this specific ID adult population, but not yet in a population sample with children [20,21].
Clinical assessment: The assessment started with observation of the participant, while (s) he was playing/interacting with his (her) mother. Subsequently, the observer took over the play and tested reactions to several toys; a toy telephone, a doll with a feeding bottle, building-blocks, a picture book, a plastic tea set and a song. The interviews and assessments were conducted in the same order by the first author (KV). All the assessments were videotaped.
Children’s behavior questionnaire (CBQ) [25]: Measures temperament on 16 subscales: activity level, anger/frustration, approach/anticipation, attentional focusing, attentional shifting, discomfort, falling reactivity and soothability, fear, high pleasure, impulsivity, inhibitory control, low pleasure, perceptual sensitivity, sadness, shyness and smiling and laughter. The questionnaire should be completed by parents on a 7-point rating scale (1=not applicable till 7=totally applicable). It is intended for children with a developmental age between 3 and 8 year. The psychometric properties are proved in a Dutch sample [26].
Social communication questionnaire: (SCQ); [27]; in Dutch: [28]) detects problems in social behavior and features of autism spectrum disorders. It is based on items of the Autism Diagnostic Interview-Revised(ADI-R) [29]. Its subcategories correspond to the three domains of autism in the DSM-diagnosis. It supplies a 40 item binary scale, which is completed by the primary caregiver(s).
The data are presented in the next section, subdivided into the following categories: adaptive behavior, temperament and maladaptive behavior.
Results
The components of adaptive behavior (VABS) are graphically represented in figure 1 as deciles scores, based on the norm group of people with a severe intellectual disability. All subjects had a severe developmental delay. A common deficit in social skills is shown for all subjects. The raw scores are presented in Table 2.
Patient 1
Patient 2
Patient 3
Patient 4
Vineland score
(developmental age in years; months)
110 (1; 7)
46 (11m)
52 (1;0)
143 (2;0)
Communication skills
41 (2;2)
10 (0;11)
15 (1;1)
49 (2;11)
Daily life skills
44 (2;0)
24 (1;4)
17 (1;1)
53 (2;6)
Social skills
25 (1;3 )
12(<0;11)
20 (0;11)
41 (1;11)
CBCL: categories in the clinical range (t-score)
Emotionally Reactive
x (80)
0
x(87,5)
x(70)
Anxious-Depressed
0
0
x (75)
x (75)
Somatic Complaints
0
0
x (87,5)
x (75)
Withdrawn
x(70)
x (85)
x (95)
x (75)
Sleep problems
0
0
x (75)
0
Attention Problems
x (85)
0
x (75)
x (80)
Agressive behavior
x (90)
0
x (70)
0
SCQ (total score)
24
22
23
26
Vineland: higher scores represent more abilities (comparable to developmental age in years; months)
CBCL: clinical scores: x= present, 0=absent, (t-scores)
SCQ: cut-off >11
Table 2: Results on the VABS, CBCL and SCQ.
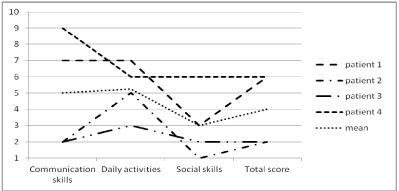
Figure 1: Results on the Vineland Adaptive Behavior Scale.
These scores are decile scores, which means that a decile score of 1 (Y-axis)
corresponds to the 10%
lowest scoring persons and a decile score of 10 (Y-axis) corresponds to the
10% highest scoring
persons. These scores are standardized for people with a severe intellectual
disability.
Results on temperament (CBQ) are shown in (Figure 2), where the mean scores of the children with KS are plotted against the mean of toddlers from the general population [25]. CBQ scores of the children with KS were remarkably low on the ‘attentional focusing’ and ‘inhibitory control’ categories and relatively high on the categories ‘low intensity pleasure’ and ‘smiling and laughter’.
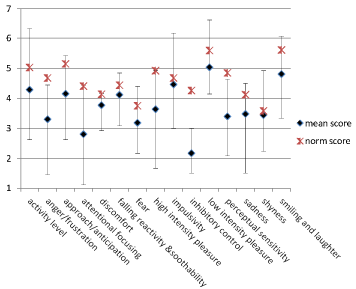
Figure 2: Results on Temperament (CBQ).
Y-as: 1= not applicable till 7= totally applicable.
Normative scores of normal developing toddlers [25].
Symptom categories of maladaptive behavior are measured by using the CBCL. All subjects scored in the clinical range for withdrawn behavior. Emotional reactivity scores and attention problems scores were also in the clinical range for patients 1, 3 & 4. The results within the clinical range are presented in Table 2, together with the raw scores. Results on the Mini PAS-ADD and clinical assessment are presented in Table 3. They show a heterogeneous but psychiatric picture. All the children fulfilled the mini PAS-ADD criteria for an Autism Spectrum Disorder, accompanied by extreme avoidance of contact in two of them and aggressive behavior in one of them. The majority of the subjects fulfilled the mini PAS-ADD criteria for Anxiety Disorder, Sleep Disorder and Mood Disorders. Psychotic symptoms in the past are reported in patient 3. All subjects scored well above the cut-off (11) on the SCQ [30].
Patient 1
Patient 2
Patient 3
Patient 4
Mini PAS-ADD categories:
Depression
0
0
x
x
Anxiety
0
0
x
0
(Hypo)mania
0
0
0
x
Psychosis
0
0
x(p)
0
Undifferented Disorder
0
0
x (p)
0
Autism Spectrum Disorder
x
x
x
x
Sleep Disorder
x
0
x
x
Clinical Assessment
Remarkable Details
Aggression
Active avoidance of contact
Active avoidance of contact
Cyclic course of mood disorder
0= absent
x= present
X (p) = present in the past
Table 3: Results on Mini PAS-ADD Interview and Clinical Assessment.
Discussion
This first study on the cognitive and neurobehavioral characteristics of children with KS strongly suggests that deficits in attention, cognitive processing and sleep are associated with a severe neuropsychiatric outcome, characterized by autism spectrum traits and mood and anxiety problems. The combined data presented in this study form a unique picture of neuro-behavioral abnormalities associated with a single but well described genetic abnormality. While the genetic abnormality of the EHMT1 locus is rare, the present study may serve as a proof of principle and template. Indeed, the number of associated genetic abnormalities, especially Copy Number Variants (CNVs) with neuropsychiatric phenotypes is rapidly growing, but only few examples exist where a single gene disruption is connected to a biological pathway and behavior. Moreover, the effects of EHMT1 mutations across species may be of help in future studies that may focus on identification of rescue mechanisms of the affected chromatin modification pathway.
Although heterogeneity is present even among the three cases with single gene disruption, interesting similarities are found. Remarkably, all subjects show deficits in social reciprocity and cognition. They all meet the criteria for Autism Spectrum Disorder (ASD) on the mini PAS-ADD. However, one has to realize that these disease categories, based on the ICD and DSM classification, give direction to the diagnostically process and are no factual diagnoses. These ICD and DSM disease categories fit people with ID as a scratchy and wrongsized coat. A definite classification should be the endpoint of the diagnostic process [16] and scores on instruments contribute to this. The high scores on autism traits are in support of recent findings from Talkowski et al [6], who described disruptions in 33 loci including EHMT1 in neuropsychiatric disorders such as ASD.
In addition, all subjects show severe developmental delay in several respects. More specific, their developmental profile, scored on the VABS, shows a relative weakness in social skills, compared to the daily living and communication skills. On the SCQ, they had high scores, in particular on lack of social reciprocity, which is in line with the results of relative weaknesses in social functioning on the VABS. In conclusion the results of the interviews, questionnaires and clinical assessment all supported the finding of striking social deficits, and all subjects fulfill clinically the criteria for ASD.
Indeed, a diagnosis of ASD in the presence of severe developmental delays should only be made when impairments in social skills outweigh the expected loss of social functioning due to Intellectual Disabilities (ID) [31]. The impairments of the patients described here, are clearly seen in social skills, especially in comparison with other genetic syndromes, like Down syndrome, Prader-Willi syndrome and Angel man syndrome [32]. Clinically, it is relevant to distinguish between intellectually disability with and without autism spectrum disorder [33]. An accurate description and diagnosis of the pattern of behavioral skills and difficulties enables professionals to optimize treatment approaches in a personalized treatment.
Besides ASD and developmental delay, sleep problems as well as attention problems are present in our cases. Sleep disturbances have been observed previously in the adult patients with EHMT mutations [15,16]. Three of the children described in our report, experience severe problems in falling asleep, often taking several hours. Also, they wake up several hours earlier than expected. While there may be some resemblance with the sleep problems in Smith Magenis syndrome, which are due to alterations in the melatonin metabolism [34], no information is yet available on the metabolism of melatonin in Kleefstra syndrome.
EHMT1 heterozygous knock-out mice showed hypo activity and autistic –like features, - less reaction to social cues, reduced exploration and higher levels of anxiety-, in comparison to wild type littermates [10]. These knock-out mice revealed reduced activity and exploration, increased anxiety while exposed to novel environments and diminished social play. In our subjects we demonstrated social withdrawnness and high levels of emotional reactivity and anxiety/ depression. The attentional and social problems observed during the clinical assessments have also been reported in adults with EHMT1 defects. A study in Drosophila identified the EHMT gene as a key regulator of cognition that orchestrates an epigenetic program featuring classic learning and memory genes [11]. The effects of this mutation on cognition were found to be reversible. This provides a challenge to develop therapeutic opportunities.
Our results should be considered in a broader perspective with genetic research in other conditions. In a large cohort of patients with schizophrenia de novo 9q34.3 deletions encompassing EHMT1 were found in three patients [35]. This indicates that deletions of EHMT1 in humans might result in highly penetrate phenotypes comprising developmental disabilities and several congenital anomalies. In addition, the EHMT1 locus is suspected to contribute to the development of psychopathology through disturbances in epigenetic mechanisms. Our findings as well as the results in adults [15,16] suggest that this is not only the case for schizophrenia, but also for a larger range of psychopathology. This supports the hypothesis that several psychiatric syndromes such as schizophrenia, autism and intellectual disability have common genetic underpinning and share a common pathway in the pathogenesis [5,36].
A limitation of this case study is the relatively small number of participants. However, even with this small number of subjects, specific neurobehavioral abnormalities such as deficits in social reciprocity and cognition, sleep disturbances and attention problems stand out. Also, these abnormalities seem to be present in EHMT mutant animal models.
We have several recommendations for future research. At first, prospective continuation of the present study can help to further elucidate the behavioral phenotype of the Kleefstra Syndrome. The question is whether these children also experience a loss of functioning later in life, like it was described in adult cases [15], and what is the course of the neurocognitive deficits? Eventually, prospective research may clarify the role of EHMT1 in neurodegenerative processes. Secondly, replication of these findings is needed in a larger cohort of participants. We recommend comparison of results of the animal studies with those in human subjects. Finally, identification of other gene disruptions that operate in the same pathway as EHMT may be highly relevant. Especially when these are combined with cognitive and social data descriptions across species.
In conclusion, our study provides a first overview of the development and behavior in four children with the KS. Their behavioral and developmental profile is characterized by a developmental delay, autism, sleep disturbances and attention problems. Deletion of the EHMT1 gene increases the risk for psychopathology in childhood, followed by a decline in functioning in adulthood. This suggests an important role in epigenetic regulation in the pathophysiological pathway of several psychiatric syndromes. Further clinical as well as fundamental research in this area may help to elucidate the precise role of EHMT1 and its clinical implications.
Acknowledgement
We thank the patients and their families for participation. This study arose from collaboration of the departments of Human genetics, Psychiatry and Karakter, child and adolescent psychiatry, of the Radboud University Medical Centre Nijmegen and Vincent van Gogh Centre of Excellence for Neuropsychiatry, Venray.
References
- Owen MJ, Craddock N, O'Donovan MC. Suggestion of roles for both common and rare risk variants in genome-wide studies of schizophrenia. Arch Gen Psychiatry. 2010; 67: 667-673.
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009; 66: 947-956.
- Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011; 168: 253-256.
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J Psychiatry. 2011; 168: 365-377.
- Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009; 32: 69-72.
- Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 149. United States: 2012 Elsevier Inc; 2012: 525-537.
- Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, et al. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. Am J Hum Genet. 2006; 79: 370-377.
- Akbarian S, Huang HS. Epigenetic regulation in human brain-focus on histone lysine methylation. Biol Psychiatry. 2009; 65: 198-203.
- Jerome TJ, Lubin FD. Histone lysine methylation: critical regulator of memory and behavior. Rev Neurosci. 2013; 24: 375-387.
- Balemans MC, Huibers MM, Eikelenboom NW, Kuipers AJ, van Summeren RC, Pijpers MM, et al. Reduced exploration, increased anxiety, and altered social behavior: Autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behav Brain Res. 2010; 208: 47-55.
- Kramer JM, Kochinke K, Oortveld MA, Marks H, Kramer D, de Jong EK, et al. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011; 9: e1000569.
- Stewart DR, Kleefstra T. The chromosome 9q sub telomere deletion syndrome. Am J Med Genet C Semin Med Genet. 2007; 145C: 383-392.
- Kleefstra T, van Zelst-Stams WA, Nillesen WM, Cormier-Daire V, Houge G, Foulds N, et al. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. J Med Genet. 2009; 46: 598-606.
- Willemsen MH, Vissers LE, Willemsen MA, van Bon BW, Kroes T, de Ligt J, et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J Med Genet. 2012; 49: 179-183.
- Verhoeven WM, Kleefstra T, Egger JI. Behavioral phenotype in the 9q subtelomeric deletion syndrome: a report about two adult patients. Am J Med Genet B Neuropsychiatry Genet. 2010; 153B: 536-541.
- Verhoeven WM, Egger JI, Vermeulen K, van de Warrenburg BP, Kleefstra T. Kleefstra syndrome in three adult patients: further delineation of the behavioral and neurological phenotype shows aspects of a neurodegenerative course. Am J Med Genet A. 2011; 155A: 2409-2415.
- Sparrow SS, Balla D.A., Cicchetti D.V. Vineland Adaptive Behavior Scales. Minneapolis: American Guidance Services; 1984.
- de Bildt A, Kraijer D, Sytema S, Minderaa R. The psychometric properties of the Vineland Adaptive Behavior Scales in children and adolescents with mental retardation. J Autism Dev Disord. 2005; 35: 53-62.
- Moss S. PH, Costello H., Simpson N., Patel P. The Mini PAS-ADD. An assessment schedule for the detection of mental health problems in adults with developmental disabilities. Manchester: University of Manchester, Hester Adrian Research Centre. 1997.
- Janssen R, Maes B. Psychometric evaluation of a Dutch version of the Mini PAS-ADD for assessing psychiatric disorders in adults with different levels of intellectual disability. J Intellect Disabil Res. 2012.
- Prosser H, Moss S, Costello H, Simpson N, Patel P, Rowe S. Reliability and validity of the Mini PAS-ADD for assessing psychiatric disorders in adults with intellectual disability. J Intellect Disabil Res. 1998; 42: 264-272.
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families. 2000.
- Verhulst FC, van der Ende J. Six-year stability of parent-reported problem behavior in an epidemiological sample. J Abnorm Child Psychol. 1992; 20: 595-610.
- Borthwick-Duffy SA, Lane KL, Widaman KF. Measuring problem behaviors in children with mental retardation: dimensions and predictors. Res Dev Disabil. 1997; 18: 415-433.
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the Children's Behavior Questionnaire. Child Dev. 2001; 72: 1394-1408.
- Sleddens EF, Kremers SP, Candel MJ, De Vries NN, Thijs C. Validating the Children's Behavior Questionnaire in Dutch children: psychometric properties and a cross-cultural comparison of factor structures. Psychol Assess. 2011; 23: 417-426.
- Rutter M, Bailey A, Lord C, Berument SK. The Social Communication Questionnaire. Los Angeles: Western Psychological Services. 2003.
- Roeyers H, Warreyn P, Raymakers R. Vragenlijst Sociale Communicatie (SCQ). Destelbergen: SIG; 2002.
- Rutter M.A. LCA, Lord C. Autism Diagnostic Interview Revised Manual. Los Angeles: Western Psychological Services. 2003.
- Allen CW, Silove N, Williams K, Hutchins P. Validity of the social communication questionnaire in assessing risk of autism in preschool children with developmental problems. J Autism Dev Disord. 2007; 37: 1272-1278.
- Towbin KE. Pervasive Developmental Disorders not otherwise Specified. Handbook of Autism and Pervasive Developmental Disorders. 3rd ed. New York: Wiley. 2005.
- Di Nuovo S, Buono S. Behavioral phenotypes of genetic syndromes with intellectual disability: comparison of adaptive profiles. Psychiatry Res. 2011; 189: 440-445.
- Howlin P. Autism and intellectual disability: diagnostic and treatment issues. J R Soc Med. 2000; 93: 351-355.
- De Leersnyder H, Claustrat B, Munnich A, Verloes A. Circadian rhythm disorder in a rare disease: Smith-Magenis syndrome. Mol Cell Endocrinol. 2006; 252: 88-91.
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012; 17: 142-153.
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004; 74: 552-557.