Abstract
Marbling, or intramuscular fat, has been consistently identified as one of the top beef quality problems. Intramuscular adipocytes distribute throughout the perimysial connective tissue of skeletal muscle and are the major site for the deposition of intramuscular fat, which is essential for the eating quality of meat. The stromal vascular fraction of the skeletal muscle contains progenitor cells that can be enhanced to differentiate to adipocytes and increase intramuscular fat.
Primary cultures of bovine intramuscular stromal vascular cells were used in this study to elucidate the effects of extracellular calcium and retinoic acid concentration on adipocyte differentiation. Cell viability assay revealed that even at different concentrations of calcium and retinoic acid, there was no significant difference on cell viability. Monitoring of the adipocyte differentiation showed that bovine intramuscular stromal vascular cells cultured in low concentration of extracellular calcium and retinoic acid had better degree of fat accumulation.
The mRNA and protein expressions of PPARγ, C/EBPα, SREBP-1c and aP2 were analyzed and showed a significant upregulation upon the reduction in the level of extracellular calcium and retinoic acid. The upregulation of these adipogenic related genes means that the decreasing concentration of calcium and retinoic acid is able to stimulate the adipogenic differentiation of bovine intramuscular stromal vascular cells. To further elucidate the effect of calcium, the expression level of calreticulin was measured. Calreticulin which is known to be an inhibitor of PPARγ was down regulated by the decreased level of calcium and retinoic acid in the culture media. The same tendency was observed on retinoic acid receptors RARα and CRABP-II. These receptors are recognized as adipogenic inhibitors; and the down regulation of their expression allowed better level of differentiation in bovine intramuscular stromal vascular cells.
Keywords: SVC; Calcium; Retinoic acid; Adipogenesis; Calreticulin; Hanwoo
Introduction
Intramuscular fat or marbling is critical for the palatability of beef. Marbling becomes a major quality problem because of the selection for high lean growth, which results in overall reduction of fat accumulation, including intramuscular fat. The amount of adipose tissue in an animal is a function of preadipocyte proliferation and differentiation. However, differences in development of these cells among adipose depots in cattle are poorly understood. The most optimal solution to this problem is to enhance intramuscular fat deposition without increasing fat deposition in other depots, which necessitates the understanding of the molecular and cellular mechanisms regulating intramuscular fat deposition [1].
It has been well established that stromal vascular cells are major sources of adipogenic cells in skeletal muscle. Enhancing adipogenesis of progenitor cells increases intramuscular fat (marbling) and improving palatability of meat. To effectively enhance adipogenesis, we must understand mechanisms regulating adipogenic differentiation of progenitor cells in the SV fraction of skeletal muscle.
Hanwoo is a type of Korean native cattle which have been raised in the Korean Peninsula since 2000 B.C. Since Hanwoo cattle have maintained stable traits through pure breeding, the current blood lineage is very valuable and is spread out mainly in the Korean Peninsula [2]. Because of this, Hanwoo is a good animal model to study adipogenesis and lipid metabolism.
This study was conducted to evaluate the effect of extracellular calcium and retinoic acid concentration on the differentiation of intramuscular bovine stromal vascular cells.
Materials and Methods
Stromal Vascular Cell (SVC) isolation
Parts of the longissimus muscle were collected directly from slaughtered castrated Hanwoo steers (15 months old). Upon collection, samples were immediately placed in a sterile ice-cold PBS with 2ng/ ml Ampothericin B and 0.2mg/ml gentamycin and transported to the laboratory. Dissected adipose tissues were cut into small pieces (approximately 2mm) and suspended in DMEM containing 2mg/ml collagenase Type 1 and 4mg/ml bovine serum albumin in a sterile 50ml conical tube. Digestion was performed for 50 minutes at 37°C with gentle shaking at a speed of 60 shakes per minute in a water bath. To stop the digestion process, an additional 5ml DMEM was added to each tube. The suspension was filtered through a 100um nylon cell strainer and centrifuged 750 X g for 10 minutes at room temperature. The medium was removed by aspiration. The pellet was washed in 10ml complete DMEM, mixed well and centrifuged again at 700 X g for 10 minutes at room temperature. The media was aspirated and resuspend the washed cells fresh DMEM media with 10% Bovine calf serum, 100U/ml penicillin and 100ug/ml streptomycin. Plate the cells and incubate at 37°C under 5% CO2.
Intramuscular SVC culture and stimulation
SVC from Hanwoo beef cattle muscle tissue were cultured to confluence in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) supplemented with 10% Bovine Calf Serum (BCS, Gibco) and 1% Penicillin/Streptomycin (Gibco) in a humidified atmosphere with 5% CO2 at 37°C. On day 2 of post-confluence (designated as day 0), cells were induced to differentiate with DMEM supplemented with 10% Fetal Bovine Serum (FBS, Gibco), 1μM dexamethasone (DEX, Sigma- Aldrich), 0.5 mM isobutylmethylxanthine (IBMX, Sigma-Aldrich), and 10μg/mL insulin (INS, Sigma-Aldrich). After 48 h (day 2), the media were replaced with DMEM supplemented with 10% FBS. The cells were subsequently re-fed every 48 h with DMEM supplemented with 10% FBS until day 14.
Treatment of calcium and retinoic acid on bovine intramuscular SVC
To investigate the effects of different concentration of calcium and retinoic acid on adipogenesis, post-confluent bovine intramuscular stromal vascular cells were treated every two days with different concentration combination of calcium (1.8 to 10 mM) and retinoic acid (0 to 8μM) for 14 days.
Cell viability assay
To determine the effect of different concentration combination of calcium and retinoic acid on bovine intramuscular SVC, a proliferation assay was performed using the CCK-8 assay (Dojindo). Cells were seeded at a density of 1 X 104 cells/well in a 96-well plate and treated with different calcium and retinoic acid concentrations. Cells were incubated for 24 and 48 hours. CCK-8 reagent was added to the cell suspension and optical density was measured at 450nm using a micro plate reader. For the treated cells, viability is expressed as the percentage of control cells.
Oil Red O staining
The extent of differentiation reflected by the amount of lipid accumulated was determined at day 14 by Oil Red O staining. Briefly, differentiated muscle tissue SVC were fixed in 10% formaldehyde in PBS for 1 hour, washed with distilled water, and dried completely. Cells were stained with 0.5% Oil Red O solution in 60:40 (v/v) isopropanol: TW for 15 minutes at room temperature washed four times with TW and dried. Differentiation was monitored under a microscope and quantified by elution with isopropanol and optical density measurements at 490 nm.
RNA extraction and RT-PCR
Confluent cultures of bovine intramuscular SVC in were seeded on 6-well plates and induced as previously described for 14 days. Total RNA was extracted from muscle tissue SVC using RNAiso Plus (Takara Shuzo Co.) according to the manufacturer’s instructions. cDNA was synthesized from1 μg of total RNA in a 20 μl reaction using a Maxime RT PreMix Kit (iNtRON Biotechnology). PCR reactions consisted of an initial denaturating cycle at 95°C for 5 minutes, followed by 30 amplification cycles: 40 seconds at 95°C, annealing for 40 seconds (temperature ranging from 56-60°C) and extension at 72°C for 1 minute. The following oligonucleotide primers were used in RT-PCR: Peroxisome proliferator-activated receptor gamma (PPAR γ) forward: CAT CTT CCA GGG GTG TCA GT; PPAR γ reverse: GGA TAT GAG GAC CCA TCC T; CCAAT-enhancer-binding protein alpha (C/EBP α) forward: GCT GAC CAG TGA CAA TGA CC; C/EBP α reverse: CTT GAC CAG GGA GCT CTC G; adipocyte protein 2 (aP2) forward: AAG CTG CAC TTC TTT CTC ACC; aP2 reverse; sterol regulatory element-binding protein-1c (SREBP-1c) forward: ACC GCT CTT CCA TCA ATG AC; SREBP-1c reverse: TTC AGC GAT TTG CTT TTG TG; Calreticulin forward: AAG TTC TAC GGT GAC GAG GAG; Calreticulin reverse: GTC GAT GTT CTG CTC ATG TTT C; retinoic acid receptor alpha (RARα) forward; GGA CAC CAA ACA TTT CCT GCC; RARα reverse: GAT GTG CTT GGT GAA GGA AGC C; cellular retinoic binding-protein II (CRAPB-II) forward: AAG GCT TTG AGG AGG AGA CC; CRAPBII reverse: TCA GGA TGA GTT CGT CGT TG; Beta-actin forward: CGC CAT GGA TGA TGA TAT TGC; Beta-actin reverse: AAG CGG CCT TGC ACA T.
Western blot analysis
Cells were seeded in 6-well plate and adipocyte differentiation was induced as described above with different concentration combination of calcium and retinoic acid. At day 14, protein was extracted by adding protein extraction solution (iNtRON Biotechnology). The lysates were clarified by centrifugation at 15000rpm for 15 min at 4 C and the protein content of the supernatant was determined using a modified Bradford assay. Diluted 30 μg of the protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose transfer membranes. The membranes were blocked with 5% skimmed milk and hybridized with the following primary Abcam™ antibodies rabbit polyclonal anti-PPARγ (ab19481), rabbit polyclonal anti-C/EBPα (ab40764), rabbit polyclonal antiaP2 (ab13979), rabbit polyclonal anti-SREBP-1c (ab3259), rabbit polyclonal anti-Calreticulin (ab2907), goat polyclonal anti-RARα (ab28767), rabbit polyclonal anti-CRABP-II (ab74365) and rabbit polyclonal anti-beta actin (ab8227). Specific proteins were identified by further incubation of the corresponding membranes with horseradish peroxidase-conjugated secondary antibodies followed by a treatment with enhanced chemiluminescence (AB Frontier). The target proteins were exposed and detected to radiographic film.
Statistical analysis
All quantitative data are representative of at least three independent experiments and the results were expressed as means + S.D. Differences between means were evaluated using ANOVA test (one-way) followed by Duncan’s Multiple Range Test. Differences were considered significant at p < 0.05. The statistical software package SAS v9.2 was used for the analysis.
Results and Discussion
Cell viability of intramuscular SVC isolated from Hanwoo beef cattle treated with different concentrations of calcium and retinoic acid
Figure 1 shows the cell viability of Hanwoo beef cattle intramuscular stromal vascular cells after treatment with different concentrations of calcium and retinoic acid for 24 and 48 hours. Data shows that the different concentration combination of calcium and retinoic acid that were used in the treatment of intramuscular SVC did not affect the viability of the cells. No significant change on the cell viability was observed between all the treatment levels of calcium and retinoic acid. Upon microscopic analysis, no change in cell morphology was observed.
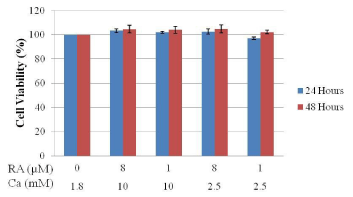
Figure 1: Effects of calcium and retinoic acid concentration on the cell
viability of SVC isolated from Hanwoo beef cattle muscle tissue incubated
for 24 and 48 hours using the CCK-8 assay. Data are means + SE of three
replicate experiments.
Differentiation of bovine intramuscular stromal vascular cells treated with different concentration of calcium and retinoic acid
Figure 2 shows the microscopic morphological changes in intramuscular stromal vascular cells treated with different concentration of calcium and retinoic acid for 14 days.
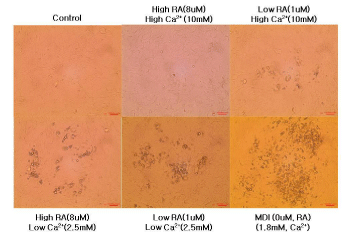
Figure 2: Differentiation of bovine intramuscular stromal vascular cells
treated with different extracellular calcium and retinoic acid concentration.
Representative microscopic morphological images of intramuscular SVC
after 14 days of differentiation.
No adipocyte differentiation was detected in the control group and intramuscular SVC cultured in 10mM calcium and 8uM. Visible amount of adipocyte differentiation started with the intramuscular SVC incubated with high calcium and low retinoic acid media, and the level of differentiation increased as the level of calcium and retinoic acid concentration decrease. These data suggests that decreasing the concentration of calcium and retinoic acid can enhance adipogenesis and promote deposition of intramuscular fat.
Effect of calcium and retinoic acid on the mRNA and protein expressions of genes associated with the differentiation of Hanwoo beef cattle intramuscular SVC
To elucidate the molecular mechanism behind the increased adipocyte differentiation of intramuscular SVC when the level of calcium and retinoic acid was decreased, several adipogenic related genes were analyzed. Data shows that as the level of calcium and retinoic acid decreased, the expression of C/EBPα, PPARγ and SREBP-1c increased. The upregulation of these transcription factors are very important in the regulation of adipogenesis.
PPARγ has always been described as a factor induced during adipocyte differentiation and is best known for its role in regulating adipogenic and lipogenic pathways. Many experiments have firmly established PPARγ as a master regulator of adipocyte differentiation [3,4]. PPARγ and C/EBPα cross regulate each other’s expression as well as governing expression of the entire adipogenic program, which includes activation of additional transcription factors [5]. The importance of the transcription factor SREBP-1c in the regulation of adipogenic differentiation has been well established in vitro. During adipogenesis SREBP-1c stimulates lipogenic gene expression and PPARγ gene expression, resulting in generating lipid droplets to store triacylglycerol in adipocytes [6]. All these three important adipogenic transcription factors were upregulated upon the reduction of the extracellular calcium and retinoic acid level. This means that the low levels of calcium and retinoic acid can stimulate adipocyte differentiation through the activation of these major adipogenic transcription factors. These data can be used in future in vivo studies where manipulation of the levels of calcium and retinoic acid can be done to promote adipogenesis and possibly intramuscular fat deposition.
The marked increase in aP2 expression during adipogenesis and the abundance of mRNA and protein in mature adipocytes established aP2 as a late marker of adipocyte differentiation and as a marker for committed preadipocytes [4,7]. In figure 3 and 4, the increased expression of aP2 upon decreasing the extracellular calcium and retinoic acid level shows that more mature adipocytes and committed preadipocytes are there compared to other intramuscular SVC treated with higher concentrations of calcium and retinoic acid. This may be a direct effect of calcium and retinoic acid on aP2 or indirectly due to the increased expression of the master regulatory transcription factor PPARγ.
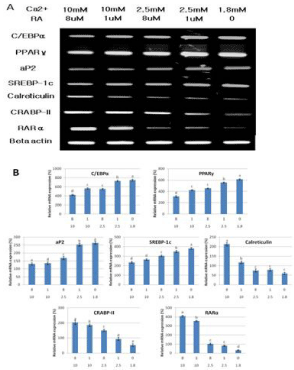
Figure 3: Changes on the mRNA expressions of genes associated with
the differentiation of Hanwoo beef cattle intramuscular stromal vascular
cells after treatment with different concentrations of calcium and retinoic
acid. (A) Representative mRNA bands. (B) Relative mRNA expression
(%).Values are expressed as means ± SE of three independent
experiments. Means with different superscript are significantly different
at p< 0.05.
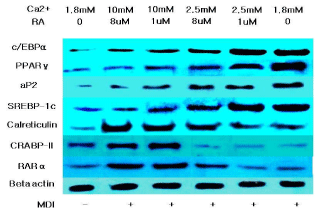
Figure 4: Western blot analysis of proteins associated with the differentiation
of bovine intramuscular stromal vascular cells after treatment with different
concentrations of calcium and retinoic acid.
A study on stem cells and 3T3-L1 pre-adipocytes shows that calreticulin could modulate adipogenesis by means of a negative feedback mechanism. PPARγ is a potent calreticulin transcription activator, since it binds to its promoter. In this way, it increases calreticulin expression, but once calreticulin is over-expressed, it inhibits cis binding of the PPARγ-RxR heterodimer to PPARγ response elements, cancelling transcriptional activation of PPARγ by fatty acids. By this mechanism, calreticulin negatively regulates both the expression of PPARγ and other critical pro-adipogenic transcription factors such as C/EBPα [8].
Calreticulin is a molecular switch that regulates cell commitment to adipocyte differentiation. Calreticulin is a multifunctional protein residing in the lumen of Endoplasmic Reticulum (ER). It maintains cellular calcium homeostasis; it is also a molecular chaperone and modulates expression of several adhesion-related genes. Calreticulinnull Embryonic Stem (ES) cells alsoexhibited a dramatic increase in adipocyte differentiation compared to calreticulin-expressing ES cells [8]. Figure 3 and 4 shows that decreasing the level of calcium and retinoic acid can reduce the mRNA and protein expression of calreticulin. This down regulation in calreticulin expression resulted in the enhancement of adipocyte differentiation due to the reduction of the inhibitory effect of calreticulin in differentiation cells.
RA regulates gene expression through two classes of nuclear hormone receptors, RA Receptors (RARs) and retinoid X Receptors (RXRs). Many of the effects of RA are due to direct binding to RAR. Xue et al. [9] presented that RAR down regulation contributes to the unresponsiveness of cells to retinoic acid and that the efficacy of retinoic acid in inhibiting adipocyte differentiation requires a threshold level of RAR. Data on the expression of RAR showed that decreasing extracellular calcium and retinoic acid down regulated the expression of RAR in bovine intramuscular SVC, which may account for the increased adipogenic differentiation.
Retinoic acid regulates gene expression by activating the nuclear RA Receptor (RAR) and its cognate intracellular lipid-binding protein CRABP-II. Berry et al. [10] showed that the diminished ability of RA to activate RAR following induction of differentiation stems from down-regulation of CRABP-II. Data demonstrate that down-regulation of CRABP-II is critical for allowing adipocyte differentiation and the expression of this protein is suppressed during adipogenesis and remains low in mature adipocytes.
Conclusion
Our research finding showed that decreasing the concentration of extracellular calcium and retinoic acid significantly promoted adipogenic differentiation in Hanwoo beef cattle intramuscular stromal vascular cells with the upregulation of important adipogenic genes. Another important finding is the suppression of calreticulin expression, which is an inhibitor of adipocyte differentiation, and the down regulation of RAR and CRABP-II. Further studies must be conducted to fully elucidate the underlying mechanism of action and to apply this knowledge in the future towards enhancing intramuscular fat deposition in beef cattle through micronutrient manipulation.
Acknowledgement
This work was carried out by the support of Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ0100232014).
References
- Albrecht E, Gotoh T, Ebara F, Xu JX, Viergutz T, Nürnberg G, et al. Cellular conditions for intramuscular fat deposition in Japanese Black and Holstein steers. Meat Sci. 2011; 89: 13-20.
- Kim JB, Lee C. Historical look at the genetic improvement in Korean cattle: Review. Asian-Australas. J Anim Sci. 2000; 13: 1467-1481.
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999; 4: 585-595.
- Dela Cruz JF, Oh YK, Hwang SG. The Control of Stromal Vascular Cell Differetiation by Retinoic Acid and Calcium in Hanwoo Beef Cattle Adipose Tissue. Journal of Animal Production Advances. 2015; 5: 835-844.
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999; 3: 151-158.
- Yoo EJ, Chung JJ, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006; 281: 6608-6615.
- Huang B, Yuan HD, Kim DY, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem. 2011; 59: 3666-3673.
- Szabo E, Qui Y, Baksh S, Michalak M, Opas M. Calreticulin inhibits commitment to adipocyte differentiation. J. Cell Biol. 2008; 182: 103-116.
- Xue JC, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARgamma. Mol Cell Biol. 1996; 16: 1567-1575.
- Berry DC, Soltanian H, Noy N. Repression of cellular retinoic acid-binding protein II during adipocyte differentiation. J Biol Chem. 2010; 285: 15324-15332.
