
Review Article
Austin Biomark Diagn. 2014;1(2): 9.
Type VI Collagen: Its Biology and Value as a Biomarker of Hepatic Fibrosis
Ki M Mak*, Priya Sehgal and Cynthia K Harris
Department of Medical Education/Center for Anatomy and Functional Morphology, Icahn School of Medicine at Mount Sinai, USA
*Corresponding author: Ki Mark Mak, Department of Medical Education/Center for Anatomy and FunctionalMorphology, Box 1007, One Gustave L. Levy Place, New York, NY, 10029, Icahn School of Medicine at Mount Sinai, New York, USA.
Received: October 27, 2014; Accepted: December 15, 2014; Published: December 16, 2014
Abstract
Collagen VI forms a filamentous network in connective tissue, linking matrix macromolecules and cells. It is composed of three chains, α1(VI), α2(VI), and α3(VI), with a globular domain at each end. Additionally, three novel chains α4, α5, and α6 were identified. Intracellularly, collagen VI monomers dimerize and form tetramers, which are secreted and associate into microfilaments extracellularly. Collagen VI gene expression is regulated differently than I or III. Collagen VI interacts with fibronectin, mediates cell adhesion and promotes migration. Soluble collagen VI acts as a sensor for tissue damage, modulating mesenchymal cell proliferation and survival, matrix homeostasis, and wound healing. Three collagen VI-deficient mouse models have been generated, which have been used to investigate collagen VI-related myopathies, mammary carcinogenesis, and skeletal muscle satellite cell homeostasis. Collagen VI is upregulated in fibrosis of liver, skin, kidneys, lungs, heart, and adipose tissue. In the liver, collagen VI normally accounts for 0.1% of total collagen, but is increased 10-fold in cirrhosis. Elevated soluble collagen VI in circulation is considered an early biomarker of alcoholic liver fibrosis. Collagen VI immunostaining is enhanced in fibrotic foci, co-distributing with collagens I, III and V. Hepatic stellate cells (HSCs) are likely the source of perisinusoidal collagen VI. The α2(VI) chain sequesters hepatic matrix metalloproteinase (MMP)-1, -3, and -8 and blocks the enzymes' activation, preventing fibrolysis. CO6-MMP, a collagen VI fragment generated by MMP-2 and -9, is a specific biomarker of collagen VI degradation in experimental liver fibrogenesis. The collagen VI receptor on HSCs offers selective targets for anti-fibrotic agents.
Keywords: Filamentous Type VI collagen; Soluble collagen VI; Collagen VI assembly; Matrix metalloproeteinase; Biomarkers of liver fibrogenesis; Hepatic stellate cells
Abbreviations
ECM: Extracellular Matrix; HSA: Human Serum Albumin; HSC: Hepatic Stellate Cell; MMP: Matrix Metalloproteinase; TGF-β: Transforming Growth Factor-β; BM: Bethlem Myopathy; MMTVPyMT: Mammary Tumor Virus-Polyoma Middle T antigen; ELISA: Enzyme-Linked Immunosorbent Assay
Introduction
The microfilamentous type VI collagen is present in most connective tissue matrices where it forms a flexible filamentous network, linking matrix macromolecules and cells. This review presents an overview of the molecular structure, biosynthesis, assembly, degradation and biological functions of collagen VI, as well as mouse models of collagen VI deficiency. In particular, we review the role of soluble collagen VI as a stimulator of cell growth, promoter of cell survival, sensor molecule for tissue damage and modulator of connective tissue matrix homeostasis. The involvement of adipose tissue-derived soluble collagen VI in mouse mammary tumorigenesis is discussed. We summarize collagen VI's regulation of the self-renewal capacity of skeletal muscle satellite cells and muscle regeneration. This review highlights the up-regulation of type VI collagen in fibrotic disease of the liver, skin, lungs, kidneys, heart and adipose tissue, and specifically provides updated information on the action of collagen VI in liver fibrogenesis and its value as a biomarker of liver fibrosis.
Structure
Type VI collagen, designated by Furthmayr et al. [1], is classified as a non-fibrillar collagen, as opposed to the interstitial fibrillar collagens I, II and III. Along with type IV collagen of the basement membrane, collagen VI is grouped under the network-forming collagens [2]. It is widely distributed in most connective tissue matrices [3-6]. Chemically, collagen VI molecule is a heterotrimeric collagenous glycoprotein made of three genetically distinct α-chains, α1(VI), α2(VI) and α3(VI). These chains differ in molecular mass: 140 kDa for the α1 and α2 chains and 250 kDa for the α3 chain [7]. The monomer consists of two globular domains at the N- and Cterminals connected by a 105 nm long triple helix [2,4,8,9]. Uniquely, the triple helical domains are extensively linked by interchange disulfide bonds that most likely endow the collagen VI molecules with a higher thermal stability as well as protease resistance. The cDNAs of the three constituent chains of human collagen VI have been cloned and a large portion of the amino acids has been sequenced [8]. Of note, there are several Gly-Y-X triplet interruptions of the amino acid sequence that are thought to provide some flexibility to the collagen VI molecules. This is in contrasting to the non-interrupted Gly-Y-X repeats for the fibrillar collagens (as in collagen I) that endow the molecules with rigidity and the fibers with mechanical strength. The flexibility of collagen VI, however, is lower than that of collagen IV, which has similar short Gly-Y-X interruptions and contains larger ones comprising up to 20 residues. Another unique structural feature of collagen VI is that it contains the sequence Arg-Gly-Asp (RGD)- dependent cell attachment sites that probably function to interact with specific cell receptors belonging to the integrin family proteins. The genes for α1(VI) and α2(VI) chains are located on chromosomes 21, and the α3(VI) gene is located on chromosome 2 [7]. The major mRNA species encoding the chains of collagen VI have sizes of 4.2 kb (α1), 3.5 kb (α2), and 8.5 kb (α3).
More recently, three novel collagen VI genes (COL6A4, COL6A5, and COL6A6 located at a single locus on human chromosome 3q22.1) that encode the α4(VI), α5(VI), and α6(VI) chains have been identified [10,11]. These chains may substitute for the α3 chains, probably forming α1α2α4, α1α2α5, and α1α2α6 heterotrimers. Unlike the α1(V), α2(V), and α3(V) subunits, these collagen VI chains display a highly restricted tissue distribution pattern [12,13], raising the possibility of tissue specific roles for the chains in collagen VI assembly and function.
Synthesis, assembly and secretion
The biosynthesis of type VI collagen was studied in cultured human fibroblasts [14] and chick embryo fibroblasts [15] using [35S] methionine metabolic labeling of cells. Two labeled polypeptides of 140 and 260 kDa were identified in the cell layer lysates, matrices and media of the human fibroblast culture, while three polypeptides of 150kDa, 140 kDa and 260 kDa were identified in the chick embryo fibroblast culture media. These give rise, after pepsin digestion, to α1(VI), α2(VI) and α3(VI), respectively. Pulse-chase experiments in the embryo chick cells indicated that more than 60% of the labeled type VI collagen was present in the culture medium after a 4-hr chase duration. In both cell systems, the amounts of polypeptides deposited extracellularly were dependent on the presence of ascorbic acid and hydroxylation of prolines and lysines in the collagenous domains, as observed in fibrillar collagens [14,16]. But, unlike the fibrillar collagens, no proteolytic processing of the N- and C-terminal domains of the polypeptide chains occurred in collagen VI biosynthesis. Another study has shown that recombinant chicken α1(VI), α2(VI) and α3(VI) collagen chains can form monomers, dimers and tetramers in NIH/3T3 cell lines. These molecules were secreted into the culture matrix, forming fibrillar meshwork [17]. This model may offer a tool for analysis of type VI collagen assembly and deposition.
The collagen VI polypeptide structure from the human fibroblast culture has been examined by electron microscopy after rotary shadowing. The images revealed that the cell layer extracts contain monomers, dimers and tetramers of collagen VI and the culture matrices contain both tetramers and multimers, while only tetramers are present in the culture media [14]. The distribution of these molecules in various compartments of the culture likely reflects the various stages of collagen VI assembly in vivo as described below. Based on the data of rotary shadowed electron microscopy, physical and biochemical analyses, the sequence of events of collagen VI's intracellular assembly has been established [1,2,9,14,15,18]. In this model, as illustrated in Figure 1, two triple helical monomers of 105 nm in length from a dimer in an anti-parallel manner with a 75 nm overlap. Two dimers associate into a tetramer, with the chains stabilized by disulfide bonds [1,7,8]. Following secretion into the ECM, the tetramers assemble into filaments by end-to-end accretion, forming thin fibrils with prominent knobs at a periodicity of about 110 nm-so-called beaded-filaments [4,14,19]. The fibrils display a width of 6-10 nm; hence, collagen VI is also described as microfilamentous [20] or microfibrillar [9].
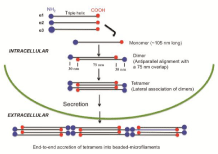
Figure 1: Model for the assembly of type VI collagen. The assembly of monomers into dimers and then into tetramers occurs intracellular. Following secretion into the extracellular matrix, the tetramers associate end-to-end into beaded-microfilaments. Note that the C-terminal globular domains contact the adjacent helices in the inner regions of the dimers that are stabilized by disulfide bonds. (Modified from [8-10]).
Gene expression
Collagen VI is abundantly expressed by cultured fibroblasts. Expression of collagen VI mRNA and its protein production were assessed in human skin fibroblast culture and the changes were compared with those of collagens I and III and fibronectin, which are known to be regulated in a coordinated fashion [21]. When the fibroblasts were grown at high densities or in a contracting collagen gel (conditions that reduce the proliferative capacity of the cells), a 2-3- fold up-regulation of the mRNA of α1(VI), α2(VI) and α3(VI) chains was observed along with an increase in corresponding proteins. There were only minimal changes for the mRNA levels of collagens I and III and fibronectin. Transformation of mouse 3T3 fibroblasts with tumor promoting phorbol esters did not change collagen VI mRNA but it did cause a 3-5-fold reduction in the mRNA levels of other matrix proteins. These data indicate that expression of α1(VI), α2(VI) and α3(VI) subunits is differently regulated in cultured fibroblasts than interstitial fibrillar collagens I and III and fibronectin. Moreover, in response to the pro-fibrotic mediator transforming growth factor-β (TGF-β), human skin fibroblasts selectively expressed the α3(VI) subunit mRNA (227% of control), while the levels of α1(VI) and α2(VI) chains were not changed [22]. Additionally, collagen VI protein was increased in the culture medium and cell layer extracts (170% of control). Therefore, the regulation of α3(VI) gene expression by TGF-β is critical for the control of collagen VI synthesis and may determine the deposition of the collagen VI molecule in the ECM. These results are compatible with a study that used recombinant type VI collagen [17].
Degradation
Intact collagen VI filaments are susceptible to degradation by serine proteinases, which are enzymes typically secreted by neutrophils and mast cells, but are resistant to both degradation by matrix metalloproteinase (MMP) -1, -2, -3 and -9 that commonly degrade other collagens, and to bacterial collagenase [23]. These properties of collagen VI suggest that it is a relatively stable molecule of the ECM, consistent with its role in matrix organization. The susceptibility of collagen VI to digestion by serine proteases suggests that collagen VI may be targeted for degradation primarily during physiological tissue turnover with inflammatory cells involvement and in early inflammatory lesions. However, Myint et al. [24] demonstrated that activated MMP-2 cleaves collagen VI extracts from normal human cornea into lower molecular weight fragments. Additionally, recent data indicate that type VI collagen can be cleared by MMP-2 and -9 in vitro with the generation of a neoepitope fragment that can be used as a marker for assessment of type VI turnover during hepatic fibrogenesis [25]. Furthermore, degradation of collagen VI has been reported for fibroblasts of periosteal explants via phagocytosis and subsequent digestion by lysosomal enzymes [26].
Biological functions
In tissue sections, the filamentous structure of type VI collagen is difficult to visualize and differentiate from other microfibrillar structures, such as fibrillin. Therefore, morphological evaluation of collagen VI organization in tissue sections or in culture samples has been carried out primarily by immuno-labeling at the light and electron microscopic levels, as well as by immunofluorescence.
In connective tissue matrices, collagen VI forms a flexible, branching filamentous network that surrounds the fibers of the major fibrillar collagens I, II and III; hence, collagen VI is sometimes called fibril-associated collagen. It anchors nerves, blood vessels, and mesenchymal cells into place, partly through interconnections with collagen IV in endothelial cell basement membranes [4,6,20]. It connects the fibrils of fibronectin in the ECM and interacts with other matrix components, including hyaluronan, decorin, syndecan, von Willebrand factor, MMPs and growth factors [27-33]. Hence, collagen VI has been called a connecting protein, linking components of the ECM [4,20].
Collagen VI and fibronectin interaction: In the ECM, the filaments of collagen VI could be observed to interconnect, but not to co-localize, at some discrete sites with fibronectin, as revealed by electron microscopy of replicas of whole mounted cultured cells and matrix [34]. Fibronectin is a multifunctional matrix glycoprotein with multiple domains that plays an important role in the interaction between cells and the surrounding ECM [35]. Collagen VI microfilaments interact with fibronectin fibrils, giving them their three-dimensional configuration. This effect was corroborated by a study that used cultured fibroblasts obtained from Col6a1 null mutant mice that lack the assembly of collagen VI and the capacity to secrete collagen VI into the ECM [36]. Consequently, the absence of collagen VI in the matrix of cultured fibroblasts resulted in a loss of the threedimensional organization of the fibronectin fibrils, which could affect various cellular functions. Additionally, an abnormal organization of fibronectin was observed in the matrix of fibroblast cultures from a patient affected by Bethlem myopathy (BM), where secretion of collagen VI was drastically reduced. In the clinic, immunofluorescent labeling of collagen VI in skin fibroblast cultures derived from BM patients has been considered a useful addition to current diagnostic services for BM [37].
Stimulation of cell growth: Soluble collagen VI, which is the pepsin-solubilized triple-helical core fragment of native collagen VI, is released from the filamentous collagen VI in the events of tissue damage and inflammation (38-42). In contrast to other collagens, soluble collagen VI stimulates proliferation of normal 3T3 fibroblasts and transformed fibrosarcoma cells in culture in the absence of growth factors [38]. The cell growth effect of collagen VI is mediated by signal transduction cascades that involve induction of tyrosine phosphorylation of proteins, including paxillin, focal adhesion kinase, and the mitogen-activated protein kinase erk2 [39]. Furthermore, these signaling cascades appear to be independent of the integrin receptor protein α2β, which mediates cell adhesion. The signaling transduction appears to require an aggregation of the collagen VI receptors or occupancy of the receptors by the native helical structure of collagen VI; interestingly, the effects can be inhibited by single chains of collagen VI (prepared from the native collagen VI by reduction and alkylation with methylene imine) [39,40].
Promotion of cell survival: Soluble collagen VI promotes survival of fibroblasts cultured in a serum-free medium through an antiapoptotic mechanism involving down regulation of the proapoptotic Bax and up regulation of cyclins A, B and D1 protein expression [41], whereas collagen I tested under the same experimental conditions has no anti-apoptotic action. The pro-survival action of collagen VI has also been seen in hepatic stellate cells (HSCs), the principal fibrogenic cells of the liver [42]. These events are mediated, in part, by the activity of transmembrane receptor NG2/chondroitin sulfate proteoglycan [40], which binds collagen VI [43]. This cellular interaction was examined by electron microscopy after rotary shadowing of a mixture of NG2 and collagen VI, which revealed an alignment of collagen VI tetramers to the central region between the two N-and C-terminal globular regions of NG2. Collagen VI also interacts with the integrin receptors. Binding of collagen VI to the integrins α1β1and α2β1 facilitates cell adhesion, spreading, and migration of smooth muscle cells and corneal fibroblasts, as well as invasion of various tumor cell lines in primary culture [44].
Sensor for tissue damage and modulator of matrix homeostasis: While the filamentous collagen VI is important in maintaining the integrity of ECM, the soluble form of collagen VI has been proposed as a sensor molecule for tissue damage, stimulating surrounding mesenchymal cell growth, promoting cell survival and wound healing. Collagen VI, along with collagens I, III, IV, and V, serves as a reservoir for cell receptors, platelet-derived growth factor, oncostatin M, MMP -1, -3, -8, -2 and -9 [31-33], and therefore regulates their availability and activity in normal tissue turnover, wound healing, and in disease. In response to needs, growth factors are released and act on nearby fibrogenic cells in the matrix, initiating cell proliferation and mediating fibrogenic activity, while MMPs act on their collagen and protein substrates, facilitating tissue turnover. For these reasons, type VI collagen is regarded as a key modulator of matrix homeostasis.
Animal models of collagen VI deficiency
Murine models of collagen V deficiency have been described, namely Col6a1 [36], Col6a3 [45], and Col6a3+/d16 (heterozygous exon 16 deletion) [46]. These animal models have been employed to investigate the molecular pathogenesis of collagen VI-related congenital Bethlem and Ullirich myopathies. Additionally, the collagen VI-knockout (Col6a1-1-) mice-in the background of the mammary tumor virus-polyoma middle T antigen (MMTV-PyMT) [47-49] - have been used as a mammary cancer model.
Specialized roles of type VI collagen in adipose tissue, mammary glands, and skeletal muscle
Collagen VI is abundantly produced and secreted by adipocytes [48,50-52]. In fact, adipose tissue is the single most abundant source of collagen VI systemically [50]. In adipose tissue, collagen VI forms an integral component of the extracellular scaffold of adipocytes and has an essential fibrogenic role in the development of obesity. The absence of collagen VI associated with collagen VI knockout mice appears to cause an unlimited expansion of individual adipocytes, but the effect is paradoxically associated with a substantial improvement of whole body energy homeostasis [52]. Expression of collagen VI is up-regulated during the progression of murine mammary tumors. Studies using Col6a1-1- mice (MMTV-PyMT mice) [47,48] have provided evidence indicating that adipocyte-derived soluble collagen VI exerts a stimulatory effect on the hyperplasia of mammary ductal epithelial cells, leading to primary tumor growth at the early stage of mammary tumorigenesis [48]. Additionally, the lack of collagen VI in knockout mice promotes apoptotic cell death of mammary epithelial cells, thereby reducing the likelihood of tumor expansion [52]. Moreover, the carboxy-terminal fragment of collagen α3(VI) chain, a proteolytic product of the full length molecule, was found to exert pro-mitogenic and pro-survival actions in part by signaling through the collagen VI binding proteoglycan NG2 receptor on the surface of malignant ductal epithelial cells [48,52]. The action leads to activation of the Akt and β- catenin signaling pathways, resulting in the mitogenic response. Therefore, collagen VI secreted by adipocytes, acting as a paracrine factor, appears to mediate a critical interaction between adipocytes and tumor cells in the tumor-stroma microenvironment. In line with these data, Park et al. [49] more recently showed that endotrophin, the C-terminal cleavage product of the α3(VI) chain derived from adipose tissue, serves as a major mediator of collagen VI-stimulated mammary tumorigenesis. Endotrophin augments mammary tumor growth and metastasis in PyMT/endotrophin mice. The effects of endotrophin on tumor growth are associated with induction of adipose tissue fibrosis, angiogenesis, inflammation and epithelial-mesenchymal transition of tumor cells. These pathologies are mediated, in part, through the up-regulated signaling pathway of TGF-β, a pro-fibrotic factor.
As an ECM protein of skeletal muscle, collagen VI is a critical component of the satellite cell niche [53]. Deficiency of collagen VI in skeletal muscle of mice is associated with muscular disorder resembling BM [36]. Investigation in collagen VIa1 null mice has shown that the lack of collagen VI causes impaired muscle regeneration accompanied by reduced capability of satellite cell to undergo self-renewal after injury to the skeletal muscle. When collagen VI is reinstated in vivo by grafting wild-type fibroblasts, the muscle stiffness associated with Col6a1-/- mice is ameliorated and the satellite cell defects in self-renewal are corrected. Thus, it was proposed that collagen VI plays a regulatory role for satellite cell homeostasis.
Type VI collagen in hepatic fibrosis
Immunohistochemistry: Immunohistological studies of the human liver revealed that type VI collagen is present in the liver lobules, stroma of portal tracts, wall of intralobular veins and Glisson's capsule [54,55]. Within the lobules, collagen VI immunostaning was either uniformly distributed in the perisinusoidal space of Disse [55] or the staining was stronger in the centrilobular and mid-lobular areas and weaker in the periportal zone [54]. Collagen VI immunoreactivity was detected in perisinusoidal HSCs by light immunohistochemistry [54] and in the HSC endoplasmic reticulum by immunoelectron microscopy [55], disclosing the cellular source of collagen VI. Figure 2 illustrates positive collagen VI staining of human HSCs (our unpublished observation). In the space of Disse, amorphous or microfibrillar materials immuno-labeled for collagen VI were observed around and between banded fibrils, suggesting that this collagen interconnects collagens I and/or III fibers (54, 55). It might be presumed that collagen VI determines the organization of the fibrillar collagens in fibrogenesis of Disse's space.
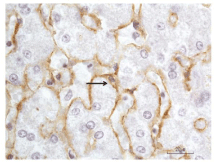
Figure 2: Immunoperoxidase staining of human hepatic stellate cells (HSCs)
for collagen VI. Liver tissue was fixed in formalin and embedded in paraffin.
The immunohistochemitry was performed as previously described [75].
Briefly, deparaffinized liver sections were treated sequentially with a rabbit
polyclonal collagen VI antibody (Novus Biologicals, Littleton, CO), anti-rabbit
polymer-horse radish peroxidase (HRP) (Dako Carpinteria, CA), and the
chromogen diaminobenzidine tetrahydrochloride to yield a brown reaction
product, with buffer washes between steps. Nuclei were counterstained with
hematoxylin. The arrow points to a positively stained perisinusoidal HSC. The
immune deposits (brown) of collagen VI could be seen in the cell body and
its cell process along the sinusoidal border. The unstained clear space in the
cytoplasm represents lipid droplets, characteristic of HSCs.
In alcoholic fibrosis and cirrhosis, intense collagen VI staining was present in the developing fibrous septa and bridging septa of cirrhotic nodules [55]. In biliary cirrhosis, strong staining for collagen VI was noted around proliferating bile ductules within the developing fibrous septa or the established septa of the cirrhotic liver [54]. Little data, however, are available on the distribution of collagen VI in progressive stages of liver fibrosis and its co-distribution with fibrillar collagens I, III and V in the human liver. Fibrosis of the liver is prevalent in elderly cadavers (mean age, 82.1±10.4 years), even when liver disease is not indicated as the cause of death [56]. In our on-going studies of the cadaveric livers with progressive stages of fibrosis, we observed an enhanced immunostaining for collagen VI in the parenchyma showing severe perisinusoidal/pericellular fibrosis, which appears to co-distribute with the increased staining for fibrillar collagens I, III or V in the fibrotic foci (Figures 3&4, unpublished data). In the developing septa and bridging septa of septal fibrosis and in the fibrotic bands of cirrhosis, the fibrous matrices show strong immunostaining for collagen VI along with collagens I, III and V (Figure 5, unpublished data). These immunohistological data point to a role for collagen VI in the integration of the fibrillar collagens in the histogenesis of fibrotic lesions, thereby contributing to the progression of hepatic fibrosis.
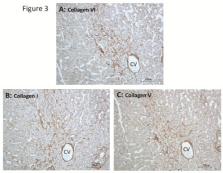
Figure 3: Co-distribution of collagens VI, I, and V in fibrotic lesion of elderly
cadaveric liver. Liver tissue was obtained from embalmed cadavers as
described in our previous studies [56,75,76]. Immunoperooxidae staining
was performed as described in Figure 2. Collagen I antibody was rabbit
polyclonal obtained from Rockland Immunochemicals (Gilbertsville, PA).
Rabbit polyclonal collagen V antibody was from Novus Biologicals (Littleton,
CO). (A), (B), and (C) are serial sections (five-µm thick) stained for collagens
VI, I, and V, respectively. Image (A) illustrates a stronger immunostaining
(brown) for collagen VI in a fibrotic area in the pericentral-mid lobular
parenchyma compared to a weaker staining reaction surrounding the lesion.
The increased collagen VI staining is coincident with an enhanced staining
for collagen I (B) and collagen V (C) in the same fibrotic area, demonstrating
co-distribution of these collagens. Note that the rim of the central vein is also
stained for these collagens. Hematoxylin counterstained. CV, central vein.
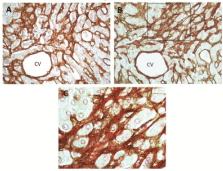
Figure 4: Co-localization of collagen VI and collagen fibers in liver fibrosis.
(A) And (B) are serial sections (five-µm thick) showing a perisinusoidal/
pericellular—chicken-wire—fibrosis in an elderly cadaveric liver. Section (A)
was immunostained for collagen VI (brown) using immunoperoxidase method
as described in Figure 2; and section (B) was immunostained for collagen VI,
followed by Sirius red staining for collagens (mainly fibrillar collagens I and
III) as previously described [56,76]. (C) Is a higher magnification view of the
boxed area in (B). It reveals localization of collagen VI immunoreactivity—
seen as darkly brown filamentous structure—to the collagen fiber bundles
stained red with Sirius red. CV, central vein.
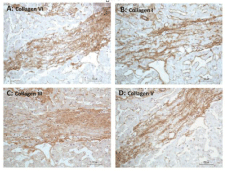
Figure 5: Immunoperoxidase staining of fibrous septum in an elderly cadaveric
liver with bridging fibrosis. (A), (B), (C), and (D) are serial sections (five-µm
thick) of a septum stained for collagens VI, I, III, and V, respectively. The
matrix of the septum is positively stained (brown) for collagens VI, I, III, and
V, suggesting co-distribution of these proteins. Hematoxylin counterstained.
In experimental fibrosis, gene expression of collagen VI was examined by in situ hybridization in conjunction with immunohistochemical detection of the protein in the liver of rats after acute CCl4 injury [57]. The α2(VI) collagen mRNA levels were elevated three days after the CCl4 treatment accompanied by up-regulation of the mRNA for collagen I. The mRNA signals for collagens VI and I were concentrated around the perivenous area with a corresponding increased staining for the protein of collagen VI. With longer duration of treatment of 14 weeks, collagen VI mRNA levels did not change, while the collagen VI protein was detected in the developing fibrous septa. It was concluded that the collagen VI gene is activated early in the fibrotic process, resulting in production of collagen VI protein. Along this line, others have described increased deposition of collagen VI along with collagens I, III and V in the matrix of developing fibrous septa and fibrotic bands of cirrhotic livers in CCl4-induced cirrhosis [58].
Biomarker of liver fibrosis: In the normal human liver, the interstitial fibrillar collagens I and III represent the most abundant collagens in the ECM, while the amount of filamentous collagen VI is low, accounting for less than 0.1% of total hepatic collagens [35,59]. In liver cirrhosis, the amount of collagen VI is increased 10-fold and its release as soluble collagen into the circulation is significant, measured by radioimmunoassay [59]. Elevated serum concentrations of collagen VI occur in chronic liver fibrotic disease irrespective of the underlying causes of liver damage, including viral hepatitis, schistosomiasis infection, children with cystic fibrosis, and alcoholic cirrhosis [60-62]. It was proposed that collagen VI serves as a predictor of liver fibrosis. Strikingly, circulating levels of collagen VI are already raised in the early stages of alcoholic liver injury [62]. Because serum collagen VI levels may reflect the activity of fibrolysis, its increase in the circulation likely represents an enhanced tissue turnover of collagen VI in the early events of hepatic fibrotic transformation and, therefore, seems to be a good indicator of early fibrogenesis. In cirrhosis, tissue collagen VI levels rose 10-fold compared to the control levels [59], while the serum concentrations of collagen VI at most doubled that of the control [60-62]. It seems that during the histogenesis of advanced fibrosis, degradation of collagen VI is impaired [59], resulting in a higher tissue concentration of collagen VI that could serve to sustain fibrogenesis by stimulation of activated HSCs or myofibroblasts for ECM production. In a report in which fibrosis markers were evaluated for predicting and diagnosing the stages of fibrosis in patients with pre-cirrhotic alcoholic liver disease, serum collagen VI levels were not correlated with the degrees of fibrosis, assessed histologically in liver biopsies [63]; however, no information was given for collagen VI assay methodology.
The value of collagen VI as a biomarker of liver fibrosis was evaluated in two rat models of hepatic fibrosis: bile duct ligation or CCl4-treatment [25]. In this investigation, a specific monoclonal antibody to CO6-MMP (a collagen type VI fragment generated by the activity of MMP-2 and -9 in vitro) was used for enzyme-linked immunosorbent assay (ELISA). It was demonstrated that CO6-MMP serum concentrations were significantly elevated and were highly associated with the histological severity of liver fibrosis in these animals. Importantly, because the CO6-MMP antibody is capable of quantifying collagen VI degradation by MMP-2 and -9, it can be employed to assess collagen VI turnover in early stages of fibrogenesis, serving as an early marker for fibrosis, which is consistent with the conclusion of previous studies that collagen VI is a good indicator of early fibrogenesis [62]. It remains to be determined whether or not MMP-2 and -9 degraded collagen VI represents a useful biomarker for the assessment of liver fibrogenesis in the clinical settings.
Type VI collagen and MMPs interaction: MMPs are critical modulators of hepatic fibrogenesis [64,65]. These proteinases are able to degrade interstitial fibrillar native collagens (MMP-1, -8 and -13), basement membrane type IV collagen and denatured fibrillar collagens (gelatinases MMP-2 and -9), non-collagenous matrix proteins and proteoglycans (stromelysin-1/MMP-3) [66]. Apart from being substrates of MMP's, collagens also sequester and modulate the availability of MMPs, particularly of the catalytic inactive proforms. As shown by immunohistochemistry, the alcoholic cirrhotic liver displays an enhanced immunostaining for filamentous collagen VI in the matrix of the fibrous septa, which appears to co-distribute with the immunoreactivity of MMP-1 and MMP-3, suggesting binding of the MMPs to collagen VI [31]. Indeed, in vitro assays revealed that the degrees of MMP binding to collagen VI correlate with the inhibition of enzymatic activities of the MMPs. The binding of MMPs to collagen VI involves specifically the α2(VI) chain. It was proposed that collagen VI, which is up-regulated in liver fibrosis, serves as a reservoir for the latent proMMPs, and that the α2(VI) chain, as a binding molecule of proMMP-1, -3, and -8, modulates the availability and activities of the MMPs by sequestering the proteinases in the ECM of the fibrotic liver. Collagen VI binding of MMPs likely conserves the proform configuration of MMPs and protects these enzymes from activation, thereby preventing matrix turnover and fibrolysis. Consequently, this biological action may perpetuate fibrous tissue deposition in the liver matrix, resulting in the progression of fibrogenesis.
Type VI collagen receptor as target for anti-fibrotic drugs: The perisinusoidal HSCs are the principal ECM producing cells of the liver. HSCs become activated in response to fibrogenic stimuli and produce increased amounts of ECM, particularly fibrillar collagens and possibly the filamentous collagen VI. Therefore, collagen VI cell surface receptors expressed on HSCs are attractive targets for anti-fibrotic agents. Because the cyclic octapeptide C*GRGDSPC*, containing the RGD sequence Arg-Gly-Asp, specifically binds to mesenchymal cells via type VI collagen receptors [5], it was used to design a specific carrier targeting HSCs in the liver. To that effect, the cyclic peptide was covalently coupled to human serum albumin (HSA), yielding pCVI-HSA [67]. The distribution of pCVI-HSA in normal and in bile duct ligation-induced fibrotic rat livers was evaluated. There was a preferential distribution of pCVI-HSA to the control normal livers and the fibrotic livers (62-75 % of the total dose) at 10 minutes after an intravenous injection. Immunohistochemical analysis revealed that 73% of the injected dose of pCVI-HSA predominantly localized to HSCs in the fibrotic liver. Importantly, in vitro studies showed that pCVI-HSA specifically bound to cultureactivated HSCs and was internalized by these cells. Therefore, pCVIHSA targeting activation-induced cell receptors may be employed as a carrier to deliver anti-fibrotic agents or drugs to HSCs to enhance the effectiveness and tissue selectivity of these factors against fibrogenesis. These findings highlight the involvement of HSC-associated collagen VI receptors in the pathogenesis of liver fibrosis.
Type VI collagen in fibrotic disease
Collagen VI is up-regulated in other fibrotic disorders as well, such as scleroderma, fibrosis of lungs, kidneys, heart and adipose tissue:
In the skin of scleroderma patients, an increased expression of α2(VI) collagen mRNA was detected by in situ hybridization, along with an up-regulation of procollagens I and III mRNA [68]. The cellular source of collagen VI mRNA could be localized to a subpopulation of fibroblasts in the dermis.
Collagen VI is present in the vascular and bronchial walls, and in the interstitial space of normal human lung biopsies [69]. Collagen VI mRNA expression is higher in lung biopsies with fibrotic changes, but the levels of expression appear not to be related to the etiologies of fibrosis. Using in situ hybridization, mRNAs for α1(VI) and α3(VI) were detected in ECM-producing myofibroblasts. Furthermore, collagen VI appears to co-distribute with collagen III in the early stage of lung fibrosis, but with collagen I in the later fibrotic stages.
Enhanced immunostaining of the kidney glomeruli for collagen VI was observed in renal fibrosis associated with the progression of diabetic glomerulosclerosis toward nodular formation [70]. There was an increased collagen VI staining in the renal fibrotic interstitium concomitant with an increased appearance of myofibroblasts, the likely cellular source of collagen VI.
Although collagen VI is a minor collagen type in the adult heart, its levels are significantly elevated in both hypertension and diabetes, conditions in which cardiac fibrosis is present and often progressive [71]. Furthermore, cardiac interstitial fibrosis and dysfunction related to hypertrophic cardiomyopathy are positively correlated with increased levels of collagen VI [72]. In cardiac post-infarction remodeling, collagen VI level increases in the infracted myocardium concurrent with an enhanced differentiation of cardiac fibroblasts to myofibroblasts, linking myofibroblast differentiation to collagen VI production [73]. Interestingly, culturing cardiac fibroblasts on collagen VI substrates induces myofibroblast differentiation but not culture on collagens I and III.
Clinically, obese human adipose tissue discloses large areas of fibrosis containing increased deposition of collagen VI accompanied by inflammatory infiltrate of activated macrophages [51]. Endotrophin, the C-terminal cleaved product of collagen α3(VI) chain, has been identified as an adipokine with potent tumorpromoting effects [49]. Recently, investigation using endotrophinover expressing transgenic mice consuming a high-fat diet, Sun et al. [74] found that endotrophin exerts a local effect on histogenesis of fibrosis in adipose tissue, leading to a systemic elevation of proinflammatory cytokines and insulin resistance in many other tissues. Blocking endotrophin with a neutralizing antibody reduces these adverse effects, emphasizing that endotrophin is a potential therapeutic target.
Conclusion and Future Direction
The structure of type VI collagen has largely been determined since its discovery thirty-one years ago, and significant advances are being made in the fields of collagen VI-related muscular disorders, mammary carcinogenesis, and fibrotic disease (in particular adipose tissue fibrosis and liver fibrosis). There are clinical data pointing to collagen VI as a marker indicative of early hepatic fibrotic changes in alcoholic patients. Experimental data demonstrate that the collagen VI receptor expressed on HSCs offers a selective target for anti-fibrotic agents, but this area has so far been under studied. The CO6-MMP ELISA for quantifying collagen VI turnover in early fibrotic stages appears to be promising, but its value as a biomarker in patients remains to be determined. Study using collagen VI-knockout mice in conjunction with induction of fibrosis-by CCl4 treatment or bile duct ligation-could help determine whether collagen VI plays a role in liver fibrogenesis. Moreover, the knockout mice could provide a source of collagen VI-deficient HSC isolates for cell culture experiments to understand the mechanism of action of collagen VI in fibrogenesis. Finally, immunohistochemistry in conjunction with immunoelectron microscopy or in situ hybridization for gene expression analysis are valuable tools for assessing collagen VI expression in normal or diseased human liver biopsies.
References
- Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983; 211: 303-311.
- Knupp C, Squire JM. Molecular packing in network-forming collagens. Adv Protein Chem. 2005; 70: 375-403.
- von der Mark H, Aumailley M, Wick G, Fleischmajer R, Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984; 142: 493-502.
- Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988; 107: 1995-2006.
- Marcelino J, McDevitt CA. Attachment of articular cartilage chondrocytes to the tissue form of type VI collagen. Biochim Biophys Acta. 1995; 1249: 180-188.
- Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997; 272: 26522-26529.
- Weil D, Mattei MG, Passage E, N'Guyen VC, Pribula-Conway D, Mann K, et al. Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am J Hum Genet. 1988; 42: 435-445.
- Chu ML, Conway D, Pan TC, Baldwin C, Mann K, Deutzmann R, et al. Amino acid sequence of the triple-helical domain of human collagen type VI. J Biol Chem. 1988; 263: 18601-18606.
- Baldock C, Sherratt MJ, Shuttleworth CA, Kielty CM. The supramolecular organization of collagen VI microfibrils. J Mol Biol. 2003; 330: 297-307.
- Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M, et al. Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem. 2008; 283: 10658-10670.
- Fitzgerald J, Rich C, Zhou FH, Hansen U. Three novel collagen VI chains, alpha4(VI), alpha5(VI), and alpha6(VI). J Biol Chem. 2008; 283: 20170-20180.
- Gara SK, Grumati P, Squarzoni S, Sabatelli P, Urciuolo A, Bonaldo P, et al. Differential and restricted expression of novel collagen VI chains in mouse. Matrix Biol. 2011; 30: 248-257.
- Sabatelli P, Gara SK, Grumati P, Urciuolo A, Gualandi F, Curci R, et al. Expression of the collagen VI a5 and a6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J Invest Dermatol. 2011; 131: 99-107.
- Engvall E, Hessle H, Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986; 102: 703-710.
- Colombatti A, Bonaldo P, Ainger K, Bressan GM, Volpin D. Biosynthesis of chick type VI collagen. I. Intracellular assembly and molecular structure. J Biol Chem. 1987; 262: 14454-14460.
- Colombatti A, Bonaldo P. Biosynthesis of chick type VI collagen. II. Processing and secretion in fibroblasts and smooth muscle cells. J Biol Chem. 1987; 262: 14461-14466.
- Colombatti A, Mucignat MT, Bonaldo P. Secretion and matrix assembly of recombinant type VI collagen. J Biol Chem. 1995; 270: 13105-13111.
- Engel J, Furthmayr H, Odermatt E, von der Mark H, Aumailley M, Fleischmajer R, et al. Structure and macromolecular organization of type VI collagen. Ann N Y Acad Sci. 1985; 460: 25-37.
- Bruns RR, Press W, Engvall E, Timpl R, Gross J. Type VI collagen in extracellular, 100-nm periodic filaments and fibrils: identification by immunoelectron microscopy. J Cell Biol. 1986; 103: 393-404.
- Amenta PS, Gil J, Martinez-Hernandez A. Connective tissue of rat lung. II: Ultrastructural localization of collagen types III, IV, and VI. J Histochem Cytochem. 1988; 36: 1167-1173.
- Hatamochi A, Aumailley M, Mauch C, Chu ML, Timpl R, Krieg T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J Biol Chem. 1989; 264: 3494-3499.
- Heckmann M, Aumailley M, Chu ML, Timpl R, Krieg T. Effect of transforming growth factor-beta on collagen type VI expression in human dermal fibroblasts. FEBS Lett. 1992; 310: 79-82.
- Kielty CM, Lees M, Shuttleworth CA, Woolley D. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem Biophys Res Commun. 1993; 191: 1230-1236.
- Myint E, Brown DJ, Ljubimov AV, Kyaw M, Kenney MC. Cleavage of human corneal type VI collagen alpha 3 chain by matrix metalloproteinase-2. Cornea. 1996; 15: 490-496.
- Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Larsen MR, Nguyen QH, et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis--identification and validation of a novel biochemical marker assay. PLoS One. 2011; 6: e24753.
- Everts V, Korper W, Niehof A, Jansen I, Beertsen W. Type VI collagen is phagocytosed by fibroblasts and digested in the lysosomal apparatus: involvement of collagenase, serine proteinases and lysosomal enzymes. Matrix Biol. 1995; 14: 665-676.
- Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992; 118: 979-990.
- Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992; 267: 5250-5256.
- Klein G, Müller CA, Tillet E, Chu ML, Timpl R. Collagen type VI in the human bone marrow microenvironment: a strong cytoadhesive component. Blood. 1995; 86: 1740-1748.
- Hoylaerts MF, Yamamoto H, Nuyts K, Vreys I, Deckmyn H, Vermylen J. von Willebrand factor binds to native collagen VI primarily via its A1 domain. Biochem J. 1997; 324 : 185-191.
- Freise C, Erben U, Muche M, Farndale R, Zeitz M, Somasundaram R, et al. The alpha 2 chain of collagen type VI sequesters latent proforms of matrix-metalloproteinases and modulates their activation and activity. Matrix Biol. 2009; 28: 480-489.
- Somasundaram R, Schuppan D. Type I, II, III, IV, V, and VI collagens serve as extracellular ligands for the isoforms of platelet-derived growth factor (AA, BB, and AB). J Biol Chem. 1996; 271: 26884-26891.
- Somasundaram R, Ruehl M, Schaefer B, Schmid M, Ackermann R, Riecken EO, et al. Interstitial collagens I, III, and VI sequester and modulate the multifunctional cytokine oncostatin M. J Biol Chem. 2002; 277: 3242-3246.
- Sabatelli P, Bonaldo P, Lattanzi G, Braghetta P, Bergamin N, Capanni C, et al. Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 2001; 20: 475-486.
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990; 10: 1-10.
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet. 1998; 7: 2135-2140.
- Hicks D, Lampe AK, Barresi R, Charlton R, Fiorillo C, Bonnemann CG, et al. A refined diagnostic algorithm for Bethlem myopathy. Neurology. 2008; 70: 1192-1199.
- Atkinson JC, Ruhl M, Becker J, Ackermann R, Schuppan D. Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro. Exp Cell Res. 1996; 228: 283-291.
- Ruhl M, Johannsen M, Atkinson J, Manski D, Sahin E, Somasundaram R, et al. Soluble collagen VI induces tyrosine phosphorylation of paxillin and focal adhesion kinase and activates the MAP kinase erk2 in fibroblasts. Exp Cell Res. 1999; 250: 548-557.
- Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001; 21: 351-372.
- Rühl M, Sahin E, Johannsen M, Somasundaram R, Manski D, Riecken EO, et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J Biol Chem. 1999; 274: 34361-34368.
- Ruehl M, Wiecher D, Sahin E, Somasundaram R, Riecken EO, Schuppan D. Soluble collagen VI as an auto paracrine inhibitor of apoptosis in hepatic stellate cells. Gastroenterology. 1999; 116: 10392.
- Tillet E, Florence Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997; 272: 10769-10776.
- Howell SJ, Doane KJ. Type VI collagen increases cell survival and prevents anti-beta 1 integrin-mediated apoptosis. Exp Cell Res. 1998; 241: 230-241.
- Pan TC, Zhang RZ, Markova D, Arita M, Zhang Y, Bogdanovich S, et al. COL6A3 protein deficiency in mice leads to muscle and tendon defects similar to human collagen VI congenital muscular dystrophy. J Biol Chem. 2013; 288: 14320-14331.
- Pan TC, Zhang RZ, Arita M, Bogdanovich S, Adams SM, Gara SK, et al. A mouse model for dominant collagen VI disorders: heterozygous deletion of Col6a3 Exon 16. J Biol Chem. 2014; 289: 10293-10307.
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992; 12: 954-961.
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005; 115: 1163-1176.
- Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012; 122: 4243-4256.
- Scherer PE, Bickel PE, Kotler M, Lodish HF. Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat Biotechnol. 1998; 16: 581-586.
- Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009; 94: 5155-5162.
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009; 29: 1575-1591.
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013; 4: 1964.
- Griffiths MR, Shepherd M, Ferrier R, Schuppan D, James OFW, Burt AD. Light microscopic and ultrastructural distribution of type VI collagen in human liver: alterations in chronic biliary disease. Histopathology. 1992; 21: 335-344.
- Loreal O, Clement B, Schuppan D, Rescan PY, Rissel M, Guillouzo A. Distribution and cellular origin of collagen VI during development and in cirrhosis. Gastroenterology. 1992; 102: 980-987.
- Mak KM, Kwong AJ, Chu E, Hoo NM. Hepatic steatosis, fibrosis, and cancer in elderly cadavers. Anat Rec (Hoboken). 2012; 295: 40-50.
- Takahara T, Sollberg S, Muona P, Uitto J. Type VI collagen gene expression in experimental liver fibrosis: quantitation and spatial distribution of mRNAs, and immunodetection of the protein. Liver. 1995; 15: 78-86.
- Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Arch A Pathol Anat Histopathol. 1993; 423: 77-84.
- Schuppan D, Ruhlmann T, Hahn EG. Radioimmunoassay for human type VI collagen and its application to tissue and body fluids. Anal Biochem. 1985; 149: 238-247.
- Shahin M, Schuppan D, Waldherr R, Risteli J, Risteli L, Savolainen ER, et al. Serum procollagen peptides and collagen type VI for the assessment of activity and degree of hepatic fibrosis in schistosomiasis and alcoholic liver disease. Hepatology. 1992; 15: 637-644.
- Gerling B, Becker M, Staab D, Schuppan D. Prediction of liver fibrosis according to serum collagen VI level in children with cystic fibrosis. N Engl J Med. 1997; 336: 1611-1612.
- Stickel F, Urbaschek R, Schuppan D, Poeschl G, Oesterling C, Conradt C, et al. Serum collagen type VI and XIV and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig Dis Sci. 2001; 46: 2025-2032.
- Lieber CS, Weiss DG, Paronetto F; Veterans Affairs Cooperative Study 391 Group. Value of fibrosis markers for staging liver fibrosis in patients with precirrhotic alcoholic liver disease. Alcohol Clin Exp Res. 2008; 32: 1031-1039.
- Arthur MJ. Collagenases and liver fibrosis. J Hepatol. 1995; 22: 43-48.
- Rojkind M. Role of metalloproteinases in liver fibrosis. Alcohol Clin Exp Res. 1999; 23: 934-939.
- Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002; 66: 19-32.
- Beljaars L, Molema G, Schuppan D, Geerts A, De Bleser PJ, Weert B, et al. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem. 2000; 275: 12743-12751.
- Peltonen J, Kahari L, Uitto J, Jimenez SA. Increased expression of type VI collagen genes in systemic sclerosis. Arthritis Rheum. 1990; 33: 1829-1835.
- Specks U, Nerlich A, Colby TV, Wiest I, Timpl R. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995; 151: 1956-1964.
- Groma V. Demonstration of collagen type VI and alpha-smooth muscle actin in renal fibrotic injury in man. Nephrol Dial Transplant. 1998; 13: 305-312.
- Kitamura M, Shimizu M, Ino H, Okeie K, Yamaguchi M, Funjno N, et al. Collagen remodeling and cardiac dysfunction in patients with hypertrophic cardiomyopathy: the significance of type III and VI collagens. Clin Cardiol. 2001; 24: 325-329.
- Spiro MJ, Crowley TJ. Increased rat myocardial type VI collagen in diabetes mellitus and hypertension. Diabetologia. 1993; 36: 93-98.
- Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, et al. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006; 290: H323-H330.
- Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014; 5: 3485.
- Mak KM, Chen LL, Lee TF. Codistribution of collagen type IV and laminin in liver fibrosis of elderly cadavers: immunohistochemical marker of perisinusoidal basement membrane formation. Anat Rec (Hoboken). 2013; 296: 953-964.
- Mak KM, Chu E, Lau KH, Kwong AJ. Liver fibrosis in elderly cadavers: localization of collagen types I, III, and IV, a-smooth muscle actin, and elastic fibers. Anat Rec (Hoboken). 2012; 295: 1159-1167.