
Review Article
Austin Biomol Open Access. 2016; 1(1): 1005.
Biomolecular Interactions and Application of Carbon Nanotubes in Nanomedicine
Zhang W1,2, Ding Q³, JinRuan J4, Fang J¹ and Ruan BH¹*
¹College of Pharmaceutical Science, Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, China
²Department of Urology, Zhejiang Cancer Hospital, China
³Department of Urology, Zhejiang Provincial People’s Hospital, China
4Hangzhou Jennifer Biotech. Inc, China
*Corresponding author: Benfang Helen Ruan, Hangzhou Jennifer Biotech. Inc, China
Received: August 10, 2016; Accepted: October 28, 2016; Published: November 01, 2016
Abstract
Modified Carbon NanoTubes (CNTs) have demonstrated early success in the field of nanomedicine. However, the progress of engineered CNTs toward clinical and preclinical trials will depend upon the outcome of safety, efficacy, and toxicological studies of CNTs. In this review, we have attempted to highlight the progress made so far, focusing on the effects of CNT surface modification, the analytical methods to measure the interactions between CNT and biomolecules, the progress in Nanomedicine and the toxicology issues that remains to be investigated.
Keywords: Carbon nanotubes; Biomolecular interaction; Nanomedicine; Toxicity; Mechanism of actions
Abbreviations
CNTs: Carbon Nano Tubes; SWCNTs: Single-Walled Carbon NanoTubes; MWCNTs: Multi-Walled Carbon Nano Tubes; BLI: Bio-Layer Interferometry; AFM: Atomic Force Microscopy; TEMTransmission Electron Microscopy; FS: Fluorescence Spectroscopy; MDS: Molecular Dynamics Stimulation; DOX: Doxorubicin
Introduction
CNTs are allotropes of carbon with a cylindrical nanostructure that was initially discovered in the late 1980s [1,2]. CNTs are members of the fullerene structural family categorized as Single- Walled Carbon NanoTubes (SWCNTs) or Multi-Walled Carbon NanoTubes (MWCNTs). Most SWCNTs are 0.4-2nm in diameter while MWCNTs are 2-100nm in diameter, but both can be millions of times longer. Using van der Waals forces p-stacking of sp2 bonds, SWCNTs and MWCNTs naturally align themselves into cylindrical forms with different “chiral” angles and radiuses that decides their unique structure and fascinating physical and chemical properties, including low density, high ductility, high mechanical strength, and excellent conductivity [3-7]. These properties led to numerous technological applications including electronic devices, field emission devices, composite materials, and important biomedical applications.
For examples, low weight (percentage) of CNTs provided significant improvements in the mechanical properties of biodegradable polymeric nanocomposites, which allowed it to be suitable scaffold materials for tissue engineering including bone, cartilage, muscles [8], cardiac [9] and nerve tissue.
The electrical conductive capacity, strong mechanical properties, and similar morphological characteristics of CNTs to neurons and neuronal structures [10] have attracted many research groups to investigate potential CNT mediated therapies for Alzheimer disease/ Parkinson disease. These therapies disrupt the disarranged protein aggregation in the nervous system, deliver functional neuroprotective growth factors, change the oxidative stress and excitotoxicity of the affected neural tissues, and regenerate the damaged neurons [11,12].
The high loading capacity and the ability to penetrate into cells without the need for an external transporter system make CNTs emerge as a potentially efficient drug delivery carrier in the biomedical and drug delivery fields [1]. Numerous research studies have been done in CNT mediated cancer drug delivery and CNT mediated thermal ablation of tumor growth in deep and poorly accessible areas (hyperthermia) [13,14].
However, undesirable side effects such as cardiopulmonary diseases, inflammation, and fibrosis [3] have been reported for several CNTs. The causes might be the differences in CNTs’ roughness, surface charge, surface group distribution [15] and affinity to various biomolecules [16]. There has been effort made to modify the CNT surface to reduce the toxicity used in cell manipulation technology [17-21] but in vivo toxicity of CNTs is still not very clear. In this review, we will focus mainly on their biomedical application and issues to be solved, so we could fulfill the promise of CNTs for nanotechnology application in biomedical science in the future.
CNT and Biomolecule Interactions
CNTs can load molecules inside their inner cavity to achieve high loading efficiency. Geometrical parameters were recognized to be important, as the most efficient host–guest interaction mechanism is achieved when the van der Waals diameter of the guest molecules matches the internal diameter of the nanotubes [22]. Additional adsorption can be in the interstitial triangular channels between the tubes, on the outer surface of the bundle (external sites), or in the grooves (major and minor) formed at the contacts between adjacent tubes, known as “endohedral filling” [23].
Unmodified CNTs contains conjugated double bonds interacting with biomolecules through van der Waals forces p-stacking of sp2 bonds. Modified CNTs contain functional groups such as carboxylate or amino group [24] that could interact with biomolecules through five interactions: hydrogen bonding, hydrophobic effects, covalent, electrostatic, and p-p stacking interactions [25].
The interactions between the CNTs and biomolecules have been studied by Atomic Force Microscopy (AFM) [26], Transmission Electron Microscopy (TEM) [27], Fluorescence Spectroscopy (FS) [28,29], Molecular Dynamics Stimulation (MDS) methods [30], and recently bio-molecular interaction assay [16]. AFM microscopic analysis provides valuable information about shape and roughness, surface charge, and surface group distribution of the modified CNTs. The Bio-Layer Interferometry (BLI)-based biomolecular interaction assay provided valuable kinetic binding information (kon, koff, KD) between CNTs and biomolecules [16]. Spectroscopy is a commonly used method to measure the binding of biomolecules to CNTs based on the concentration difference before or after CNT bindings [31], but at high protein concentration, the subtraction method might have huge experimental errors and result in false positives.
For examples, we recently measured the interaction between CNT and proteins [16] using multiple analytical approaches. The biomolecular interaction assay demonstrated that Wheat Germ Agglutinin (WGA) protein binds extremely tightly to the carbonated MWCNTs (f-MWCNT) but not to the unmodified MWCNTs (p-MWCNT). However, the spectroscopy measurement of WGA concentration in solution before or after CNT bindings, p-MWCNT appeared to have higher binding capacity and f-MWCNT showed a 2 fold better binding affinity; the apparent contradiction between the spectroscopy results and BLI data could explained by the ability of spectroscopy subtraction method fails to differentiate the specific from the non-specific interactions, and also the off-rate of a WGA to CNTs cannot be measured directly using the spectroscopic method. The tight binding of the f-MWCNT to WGA was discovered because after extensively washing the WGA-f-MWCNT complex by water for 5 times, WGA could still bind to f-MWCNT beads as shown in Bradford assay [16]. However, it is labor intensive to wash tens of fractions for 5 times manually.
Interestingly, CNTs are known to be extremely effective carriers of proteins, peptides, nucleic acids, and small molecular drugs for delivery into living cells. Recently, however, biomolecular interaction analysis by Forte Bio and Plexera methods demonstrated that the binding affinities to CNTs are dependent on its surface modifications [16]. In Plexera assay, CNT was cross-linked on a chip and biomolecule binding was detected by Surface Plasmon Resonance (SPR) technology. In Forte Bio assay, CNT was absorbed on a chip and biomolecule binding was detected by Bio-Layer Interferometry (BLI)-based techno technology. Both assay showed that nonfunctional CNT (p-MWCNTs)could not bind to proteins effectively, whereas the carboxylated functional CNT (f-MWCNTs) bound to proteins extremely tightly with a very small off-rate with its binding KD to WGA at 4.6×10-11 M and FKBP12 at 3.2×10-9 M [16].
Since the kinetic data of CNTs binding to biomolecules (proteins, peptides, nucleic acids, and small molecular drugs) are rare, we extended our early kinetic study [16] and measured more kon, koff and KD values of f-MWCNT or p-MWCNT binding to various biomolecules. Figure 1 showed binding data collected using Plexera instrument and Figure 2 using ForteBio K2 instrument.
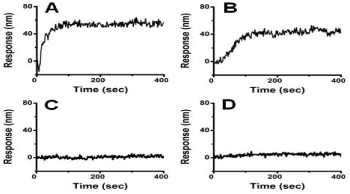
Figure 1: Plexera Instrument measurement of kon, koff, KD; sensorgrams show
tight binding of f-MWCNT to FKBP52 and KGA; but essentially no binding
of p-MWCNT to FKBP52 or KGA. (A) 57.7 μM FKBP52 in PBS binding to
f-MWCNT with kon: 4.1×103 1/Ms; koff: 1.33×10-2 1/s; KD:3.25×10-6 M; (B) 8.3
μM KGA in PBS binding to f-MWCNT with kon: 9.09×103 1/Ms; koff: 1.57×10-1
1/s; KD:1.73×10-5 M; (C) 57.7 μM FKBP52 in PBS with no significant binding
to p-MWCNT;(D)Essentially no binding of 8.3 μM KGA in PBS to p-MWCNT.
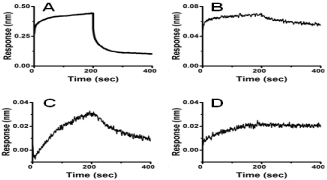
Figure 2: 2: Fortebio K2 Instrument measurement of kon,
koff, KD; sensorgrams show f-MWCNT binding of DOX,
Ebselen, BSA and ssDNA. (A) 10 μM Doxorubicin in PBS
with kon: 8.06×104 1/Ms; koff: 1.45×10-2 1/s;KD: 1.81×10-7 M;
(B) 0.5 μM ssDNA in PBS obtaining kon: 1.55×106 1/Ms;
koff: 1.01×10-3 1/s;KD:6.5×10-10 M; (C) 10 μM Ebselen in
PBST-5% DMSO gaining kon: 4.19×102 1/Ms; koff: 5.52×10-
3 1/s; KD: 1.32×10-5 M; (D) 0.5 μM BSA in PB Shaving kon:
5.2×104 1/Ms; koff: 2.5×10-4 1/s; KD: 4.82×10-9 M.
As shown in Figure 1 in the Plexera assay where both f-MWCNT and p-MWCNT are cross-linked on the chip, the f-MWCNT showed strong binding to random proteins such as KGA andFKBP52 with KD of 3.2×10-6 M, 1.7×10-5 M, respectively; however, p-MWCNT showed essentially no binding to any of these proteins.
Interestingly, as shown in Figure 2 in ForteBio assay, the binding modes of f-MWCNT to various biomolecules are different. The f-MWCNT could bind to proteins and DNA strongly and nonselectively. Strong dose dependent binding to random proteins such as BSA, WGA [16], and FKBP12 [16] were observed with KD of 4.82×10-9 M, 2×10-8 M, 6×10-9 M, respectively. The ssDNA with 20 base pairs could also bind to f-MWCNT very strongly (KD: 6.5×10- 10 M) with very small off-rate of koff: 1.01×10-3 1/s. Since proteins and DNA are important macromolecules for biological functions, the random and tight binding are likely to inhibit DNA replication, transcription, translation, and cellular signaling; this could contribute to the in vivo cytotoxicity.
In addition, although CNT was reported to have high binding capacity for small drug molecules, ForteBio assay (Figure 2) showed that both water-soluble Doxorubicin (DOX) and lipophilic Ebselen have a quick off-rate with KD of 1.81×10-7 M and 1.32×10-5 M, respectively. Therefore, in order to use f-MWCNT as a vehicle for Doxorubicin (DOX) and Ebselen drug delivery, a cross-link between the f-MWCNT and the drug must be made to keep both together in in vivo biological system.
Taken together, these results showed that not only the shape and size are important for CNT functions, but also the modification on CNT could totally change the property and the toxicity of the materials. The f-MWCNT showed essentially no selectivity among various polypeptide and proteins, and is likely to bind to any proteins, DNA and lipids; this might explain why CNT is able to penetrate membrane directly [28] and could inhibit Calcium channels [29]. To predict the in vivo biocompatibility, novel CNTs are highly recommended to test the kinetic binding to biomolecules such as proteins, DNA, RNA and cofactors.
CNT Surface Modification
Biomolecular interaction analysis demonstrated that CNT surface modification might determine the property of the modified materials [16]. Unmodified CNT showed good biocompatibility because it does not show strong interaction with biomolecules. However, the carboxylated CNT binds to various biomolecules tightly and nonselectively and resulted in poor biocompatibility such as hemolysis and inhibition of cell proliferation[16].
To reduce toxicity, various CNT surface modifications have been prepared [34,35]. For example, pulmonary surfactant was coated on CNTs to improve their chemical properties for biological application [36]. Perfluorooctanesulfonyl fluoride was used to form a super hydrophobic surface that provided CNTs with enhanced antibacterial and mechanical properties [37]. Using the layer self-assembly technique, laminin CNTs formed basement membranes that could successfully induce the differentiation and maturation of the neural stem cells [38]. Primary rat hippocampal neurons that were cultured on 4-hydroxynonenal protein coated CNT basement grew a greater number of axons and branches than those in the control group [39]. In addition, serum-coated CNTs could significantly decrease the CNT toxicity [26], whereas WGA-modified CNT could reduce hemolysis and increase its inhibition of cancer cell growth [16].
Current hot research field is to modify CNT for targeted drug delivery. Both Folate-decorated magnetic MWCNTs [40] and pluronic-F108 surfactant-wrapped CDDP-encapsulated ultra short SWCNTs (US-tube) [41] were prepared; when loaded with Doxorubicin (DOX), both modified CNTs demonstrated enhanced cytotoxicity toward cancer cell lines than free DOX in both a dose- and a time-dependent manner. Thiol-modified siRNA cargo molecules were engineered to link to the amine functional groups on the sidewalls of phospholipid–polyethylene glycol–SWCNTs via cleavable disulfide bonds; these disulfide linkages facilitate the release of the cargo molecules from the SWCNT conjugate upon cellular uptake [42]. Recently, antibody-conjugated multi-walled carbon nanotubes were reported suitable for targeted ultrasound imaging and drug delivery [43].
These experiment data demonstrated that after surface modifications, CNTs obtained additional property of its coating materials.
CNT in Anti-Cancer Drug Delivery
Preclinical in vitro and in vivo results showed early promise of CNT as nanocarriers for cancer treatment [44-48], but no FDA approvals or clinical trials up to date has been reported. Nanocarriers, once in the vicinity of the tumor, can:
(i) release their cytotoxic content next to the cancer cells; (ii) bind to the membrane of the cancer cells and release their content in a sustained way; (iii) be internalized into the cells [49].
CNTs are completely insoluble in all solvents. Therefore, CNTs need to be shortened in length and diameter and then chemically modified to transform into water-soluble carriers, which increases their biocompatibility and decreases their toxicity [50-52]. These novel modified CNTs can cross the plasma membrane and enter into the cancerous cells by endocytosis or direct penetration [53,54]. In addition, surface modification using cancer cell targeting molecules, provides CNT with enhanced tumor cell specificity which could overcome the cytotoxicity and the multidrug resistance issues [55- 60].
For example, the sidewall of SWCNTs was conjugated to a firstline cytotoxic agent (cisplatin) and a targeting moiety (Epidermal Growth Factor, EGF); the resulting nanocarrier showed an enhanced efficacy in inhibiting squamous cancer cells that have Epidermal Growth Factor Receptor (EGFR) over expressed, accumulating CNT in the tumor tissue, and causing reduction in tumor size [61,62]. Also, a water-soluble SWCNT conjugated with docetaxel and NGR (Asn-Gly-Arg) peptide was reported to enhance in vitro and in vivo efficacy in the PC3 cell line and in a murine S180cancer model [63]. In addition, several targeted conjugates were prepared to deliver doxorubicin specifically to tumor tissue. The folic acid conjugated chitosan modified CNT could selectively deliver DOX to SMMC-7721 hepatocellular carcinoma cells; the nanocarrier killed the HCC SMMC-7721 cell, minimized the tumor growth in nudemice xenograph model, enhanced pharmaceutical efficiency and decreased in vivo toxicity [64]. The MWCNT conjugated to doxorubicinloadedhyaluronic acid could be internalized specifically by A549 human lung adenocarcinoma cells via hyaluronan receptors; this resulted in improved potency and apoptotic activity in cell-based assay, tumor growth inhibition in Ehlrich ascites tumor bearing mice model and in breast cancer-induced rat model, and essentially no toxicity in mice and rats [65]. The doxorubicin-loaded PEGylated and angiopep-2 conjugated MWCNT was tested for the treatment of brain glioma; the modified nanocarrier demonstrated superior anti-glioma effect in vitro by inhibiting C6 glioma cells and in vivo by increasing in the median survival time of mice with glioma [66]. In addition, gemcitabine-loaded folic acid-conjugated CNT was shown to release sustainly gemcitabine at the lysosomal pH in MCF-7 tumor; appreciable gemcitabine release was observed in the systemic circulation, but a lot less in liver, kidney, spleen and lungs [67]. Taken together, the in vivo pharmacokinetic parameters demonstrated that drug-loaded active-target-conjugated CNT could enhance residence time and half-life with respect to the free drug and the non-targeted system
CNT Toxicity and Potential Mechanism of Actions
CNT showed great promise in biomedical application, and thus the CNT toxicity evaluation become extremely important. As an insoluble material, the toxicity of CNTs is not easy to be investigated. Non-functionalized CNTs was reported to decrease cell viability, and an inflammatory response has been observed with pristine CNTs [68]. Since CNTs have large surface areas to absorb chemicals, irreversible depletion of the nutrients in the culture solution was proposed as a mechanism to inhibit cell growth. Another reason is that CNTs could contain residual chemicals from preparations that might inhibit cell proliferation in a dose and time-dependent manner.
Limited research has shown that CNT can penetrate into the body through intravenous, dermal, subcutaneous, inhalational, intraperitoneal, or oral routes [69]. The inhaled CNTs (0.5 mg) could encounter macrophage and form granuloma; furthermore, CNT aggregation via van der Waals’ interactions could stimulate profibrogenic cellular responses and result in the pulmonary toxicity in vivo.
Recently, CNTs were reported to stimulate strong cellular response, not only based on their similarity to the surface roughness and the diameter of the neural cells [70], but also throughcalcium channels [71].We discovered that on the basis of the results shown in (Figure 1,2), carboxylated CNTs bind strongly and non-specifically to important biomolecules such as proteins, DNA, RNA, and small molecules; these molecules are delicately balanced to maintain important signaling pathways and normal functions of cellular machinery. Once the CNTs enter the cells though endocytosis or direct penetration, these CNTs could bind to biomolecules, interrupt the cellular machinery, and result in oxidative stress, which is recognized as the main mechanism of CNT toxicity [72].
To make the toxicity and inflammation situation even worse, CNT degradation is very slow. After respiratory exposure, only 18% of instilled CNT was eliminated in the lungs after 60 days [73]. The calculated biological half clearance time of CNT was around 50 days [74]. The biodegradation routes of CNT are still poorly understood. However, limited in vitro experiment results suggested that MPO mediated radical mechanism might involve with CNT biodegradation pathway [75].
Concluding Remarks and Future Perspective
CNTs have unique structure and fascinating physical and chemical properties. After various modifications, CNTs have demonstrated early success in nanomedicines to advance pharmacokinetics and drug delivery, which might be able to address problems in central nervous system pathologies and Multi Drug Resistant (MDR) cancer therapeutics. In our view, multifunctional CNTs will continue to receive significant research attention because of their unique physicochemical properties in targeted and controlled drug delivery. However, the use of CNTs in therapeutics and disease diagnosis needs to be evaluated by systematic determination of the benefit and the risk. The progress of engineered CNTs toward clinical and preclinical trials will depend upon the outcome of safety, efficacy, and toxicological studies of CNTs.
Biocompatibility of CNTs is largely determined by its surface modifications, which interacts with bio macromolecules in cells and tissues. AFM, TEM, FS, MDS, BLI and SPR are advanced technologies that provide critical information of the interactions between modified CNTs and bio macromolecules, which can be used to determine preliminary CNT biocompatibility issues. However, for further safety evaluation, both the in vivo metabolic profiling (ADME) of CNT and the mechanism of toxicity are critical information that is still poorly understood. Currently, the major hurdle in pharmacokinetic investigation is the insolubility of CNTs. Effort has been made to explore methods to increase the solubility of CNTs which including shortening the length dramatically, adding more amine groups or other water-soluble groups.
In this article, we have attempted to highlight the progress made so far, focusing on the effect of CNT surface modification, the interactions between CNT and biomolecules, nanomedicine and toxicity studies, as shown in Figure 3, looking into the future, modified multifunctional CNTs as nanomedicine might provide cutting-edge technology for cancer theranostics, targeted cancer therapy and imaging diagnosis to monitor the treatment in real time. However, in vivo toxicity studies are imminent and critical in order to transform carbon nanotubes into a clinical reality.
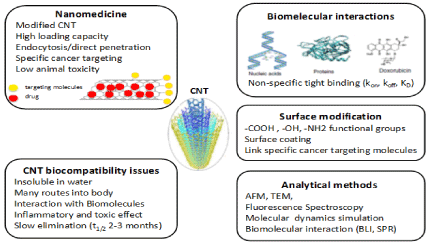
Figure 3: CNTs showed great promise as nanomedicine, but the biocompatible and toxicological investigations of CNTs
will have to take place simultaneously to transform carbon nanotubes into a clinical reality.
References
- Kim KY. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine. 2007; 3: 103-110.
- Zhao X, Liu R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ Int. 2012; 40: 244-255.
- Sethuraman A, Stroscio MA, Dutta M. Potential Applications of Carbon Nanotubes in Bioengineering. Biological Nanostructures and Applications of Nanostructures in Biology.2004; 51-68.
- Yao Z, Postma C, Balents L, Dekker C. Carbon nanotube intramolecular junctions. Letters to Nature. 1999; 402: 273-276.
- Fuhrer MS, Nygard J, Shih L, Forero M, Yoon YG, Mazzoni MSC, et al. Crossed Nanotube Junctions. Science. 2000; 288: 494-497.
- Rueck T, Kim K, Joselevich E, Tseng GY, Cheung CL, Lieber CM. Carbon Nanotube-Based Nonvolatile Random Access Memory for Molecular Computing. Science. 2000; 289: 94-97.
- Wei W, Sethuraman A, Jin C, Monteiroriviere NA, Narayan RJ. Biological properties of carbon nanotubes. J Nanosci Nanotechnol. 2007; 7: 1284-1297.
- Shin SR, Shin C, Memic A, Shadmehr S, Miscuglio M, Jung HY, et al. Aligned Carbon Nanotube-Based Flexible Gel Substrates for Engineering Biohybrid Tissue Actuators. Advanced Functional Materials. 2015; 25: 4486-4495.
- Ahadian S, Yamada S, Ramon-Azcón J, Estili M, Liang X, Nakajima K, et al. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016; 31: 134-143.
- Mylvaganam K. Carbon nanotubes build better protective body armor. Advanced Materials and Processes. 2008; 166: 24.
- Wang K, Tang RY, Zhao XB, Li JJ, Lang YR, Jiang XX, et al. Covalent bonding of YIGSR and RGD to PEDOT/PSS/MWCNT-COOH composite material to improve the neural interface. Nanoscale. 2015; 7: 18677-18685.
- Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P, et al. Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert Opinion on Drug Delivery. 2015; 12: 1089-1105.
- Raza K, Kumar D, Kiran C, Kumar M, Guru SK, Kumar P, et al. Conjugation of Docetaxel with Multiwalled Carbon Nanotubes and Codelivery with Piperine: Implications on Pharmacokinetic Profile and Anticancer Activity. Molecular Pharmaceutics. 2016; 13: 2423-2432.
- Fabbro C, Ali-Boucetta H, Ros TD, Kostarelos K, Bianco A, Prato M. Targeting carbon nanotubes against cancer. Chemical Communications. 2012; 48: 3911-3926.
- Wirth M, Fuchs A, Wolf M, Ertl B, Gabor F. Lectin-Mediated Drug Targeting: Preparation, Binding Characteristics, and Antiproliferative Activity of Wheat Germ Agglutinin Conjugated Doxorubicin on Caco-2 cells. Pharmaceutical Research. 1998; 15: 1031-1037.
- Chen X, Fang J, Cheng Y, Zheng J, Zhang J, Chen T, et al. Biomolecular interaction analysis for carbon nanotubes and for biocompatibility prediction. Analytical Biochemistry. 2016; 505: 1-7.
- Buchoux J, Aime JP, Boisgard R, Nguyen CV, Buchaillot L, Marsaudon S. Investigation of the carbon nanotube AFM tip contacts: free sliding versus pinned contact. Nanotechnology. 2009; 20: 475701.
- Narui Y, Ceres DM, Chen J, Giapis KP, Collier CP. High aspect ratio silicon dioxide-coated single-walled carbon nanotube scanning probe nanoelectrodes. Journal Physical Chemistry. 2009; 113: 6815-6820.
- Stevens R.M. New carbon nanotube AFM probe technology. Materials today. 2009; 12: 42-45.
- Suga H, Naitoh Y, Tanaka M, Horikawa M, Kobori H, Shimizu T. Nanomanipulation of Single Nanoparticle Using a Carbon Nanotube Probe in a Scanning Electron Microscope. Applied Physics Express. 2009; 2: 055004-0550043.
- Zhang M, Li J. Carbon nanotube in different shapes. Materials today. 2009; 12: 12-18.
- Wang R, Yu B, Jiang X, Yin J. Understanding the Host–Guest Interaction Between Responsive Core-Crosslinked Hybrid Nanoparticles of Hyperbranched Poly(ether amine) and Dyes: The Selective Adsorption and Smart Separation of Dyes in Water. Advanced Functional Materials. 2012; 22: 2606-2616.
- Pasinetti PM, Roma F, Riccardo JL, Ramirez-Pastor A.J. Statistical Thermodynamics and Surface Phase Transitions of Interacting Particles Adsorbed on One-Dimensional Channels Arranged in a Triangular Cross-Sectional Structure. Solid State Phenomena. 2009; 150: 73-100.
- Li X, Chen W, Zhan Q, Dai L, Sowards L, Pender M, et al. Direct Measurements of Interactions between Polypeptides and Carbon Nanotubes. The Journal of Physical Chemistry B. 2006; 110: 12621-12625.
- Ezzeddine A, Chen Z, Schanze KS, Khashab NM. Surface Modification of Multiwalled Carbon Nanotubes with Cationic Conjugated Polyelectrolytes: Fundamental Interactions and Intercalation into Conductive Poly(methyl methacrylate) composites. ACS Applied Materials Interfaces. 2015; 7: 12903-12913.
- Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proceedings of the National Academy of Science of the United States of America. 2011; 108: 16968-16973.
- Liu Y, Huang J, Sun MJ, Yu JC, Chen YL, Zhang YQ, et al. A fluorescence-Raman dual-imaging platform based on complexes of conjugated polymers and carbon nanotubes. Nanoscale. 2014; 6: 1480-1489.
- Li X, Chen W, Zhan QW, Dai L.M, Sowards L, Pender M, et al. Direct measurements of interactions between polypeptides and carbon nanotubes. The Journal of Physical Chemistry B. 2006; 11: 12621-12625.
- Sen F, Boghossian AA, Sen S, Ulissi ZW, Zhang J, Strano MS. Observation of Oscillatory Surface Reactions of Riboflavin, Trolox, and Singlet Oxygen Using Single Carbon Nanotube Fluorescence Spectroscopy. ACS Nano. 2012; 6: 10632-10645.
- Byrne EM, McCarthy MA, Xia Z, Curtin WA. Multiwall Nanotubes Can Be Stronger than Single Wall Nanotubes and Implications for Nanocomposite Design. Physical Review Letter. 2009; 103: 045502-045505.
- Takada T, Kurosaki R, Konno Y, Abe S. Interaction of multi-walled carbon nanotubes with water-soluble proteins: effect of sidewall carboxylation. J Nanosci Nanotechnol. 2014; 14: 3216-3220.
- Schrlau MG, Bau HH. Carbon-based nanoprobes for cell biology. Microfluidics & Nanofluidics, 2009; 7: 439-450.
- Park KH, Chhowalla M, Iqbal Z, Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. Journal of Biological Chemistry. 2003; 278: 50212-50216.
- Monaco AM, Giugliano M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014; 5: 1849-1863.
- Prato M, Kostarelos K, Bianco A. Functionalized Carbon Nanotubes in Drug Design and Discovery. Accounts Chemical Research. 2008; 41: 60-68.
- Gasser M, Wick P, Clift MJ, Blank F, Diener L, Yan B, et al. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Particle and Fibre Toxicology. 2012; 9: 1-13.
- Song K, Gao A, Cheng X, Xie K. Preparation of the superhydrophobic nano-hybrid membrane containing carbon nanotube based on chitosan and its antibacterial activity. Carbohydrate Polymers. 2015; 130: 381-387.
- Kam NW, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009; 9: 273-278.
- Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neural growth. J Mol Neurosci. 2000; 14: 175-182.
- Lu YJ, Wei KC, Ma CC, Yang SY, Chen JP. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B Biointerfaces. 2012; 89: 1-9.
- Guven A, Rusakova IA, Lewis MT, Wilson LJ. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials. 2012; 33: 1455-1461.
- Samori C, Ali-Boucetta H, Sainz R, Guo C, Toma FM, Fabbro C, et al. Enhanced anticancer activity of multi-walled carbon nanotube-methotrexate conjugates using cleavable linkers. Chemical Communications. 2010; 46: 1494-1496.
- Wu H, Shi H, Zhang H, Wang X, Yang Y, Yu C, et al. Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials. 2014; 35: 5369-5380.
- Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nature Nanotechnology. 2009; 4: 627-633.
- Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008; 68: 6652-6660.
- Liu Z, Fan AC, Rakhra K, Sherlock S, Goodwin A, Chen X, et al. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew Chem Int Ed Engl. 2009; 48: 7668-7672.
- Prakash S, Malhotra M, Shao W, Tomaro-Duchesneau C, Abbasi S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv Drug Deliv Rev. 2011; 63: 1340-1351.
- Adeli M, Soleyman R, Beiranvand, Z, Madani F. Carbon nanotubes in cancertherapy: a more precise look at the role of carbon nanotube-polymer interactions. Chem Soc Rev. 2013; 42: 5231-5256.
- Peer D, Karp JM, Hong S, Farokhzad O.C, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007; 2: 751-760.
- Mu QX, Liu W, Xing YH, Zhou HY, Li Z, Zhang Y, et al. Protein Binding by Functionalized Multiwalled Carbon Nanotubes is Governed by the Surface Chemistry of Both Parties and the Nanotube Diameter. The Journal of Physical Chemistry C. 2008; 112: 3300-3307.
- Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano. 2007; 1: 50-56.
- Ali-Boucetta H, Al-Jamal KT, Muller KH, Li S, Porter AE, Eddaoudi A, et al. Cellular uptake and cytotoxic impact of chemically functionalized and polymer-coated carbon nanotubes. Small. 2011; 7: 3230-3238.
- Wu W, Li R, Bian X, Zhu Z, Ding D, Li X, et al. Covalently combining carbon nanotubes with anticancer agent: preparation and antitumor activity. ACS Nano. 2009; 3: 2740-2750.
- Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, Luangsivilay J, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nature Nanotechnology. 2007; 2: 108-113.
- Fabbro C, Ali-Boucetta H, DaRos T, Kostarelos K, Bianco A, Prato M. Targeting carbon nanotubes against cancer. Chemical Communications. 2012; 48: 3911-3926.
- Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotechnology. 2007; 2: 47-52.
- Heister E, Neves V, Tilmaciu C, Lipert K, Sanz V, Coley HM, et al. Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon. 2009; 47: 2152-2160.
- Zhang X, Meng L, Lu Q, Fei Z, Dyson P. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009; 30: 6041-6047.
- Li R, Wu R, Zhao L, Wu M, Yang L, Zou H P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano. 2010; 4: 1399-1408.
- Cheng J, Meziani MJ, Sun YP, Cheng SH. Poly(ethylene glycol)-conjugated multi-walled carbon nanotubes as an efficient drug carrier for overcoming multidrug resistance. Toxicol Appl Pharmacol. 2010; 250: 184-193.
- Bhirde AA, Patel S, Sousa AA, Patel V, Molinolo AA, Ji Y, et al. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine. 2010; 5: 1535-1546.
- Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, et al. Targeted Killing of Cancer Cells in Vivo and in Vitro with EGF-Directed Carbon Nanotube-Based Drug Delivery. ACS Nano. 2009; 3: 307-316.
- Wang L, Zhang M, Zhang N, Shi J, Zhang H, Li M, et al. Synergistic enhancement of cancer therapy using a combination of docetaxel and photothermal ablation induced by single-walled carbon nanotubes. Int J Nanomedicine. 2011; 6: 2641-2652.
- Ji Z, Lin G, Lu Q, Meng L, Shen X, Dong L, et al. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J Colloid Interface Sci. 2012; 365: 143-149.
- Datir SR, Das M, Singh RP, Jain S. Hyaluronate tethered, “smart” multiwalled carbon nanotubes for tumor-targeted delivery of doxorubicin. Bioconjugate Chem. 2012; 23: 2201-2213.
- Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012; 33: 3324-3333.
- Singh R, Mehra NK, Jain V, Jain NK. Gemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cells. J Drug Targeting. 2013; 21: 581-592.
- Rodriguez-Yanez Y, Munoz B, Albores A. Mechanisms of toxicity by carbon nanotubes. Toxicol Mech Methods. 2013; 23: 178-195.
- Kam NW, Liu Z, Dai H. Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl. 2006; 45: 577-581.
- Sorkin R, Gabay T, Blinder P, Baranes D, Ben-Jacob E, Hanein Y. Compact self-wiring in cultured neural networks. J Neural Eng. 2006; 3: 95-101.
- Geng J, Kim K, Zhang J, Escalada A, Tunuguntla R, Comolli L.R, et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature. 2014; 514: 612-615.
- Shvedova AA, Fabisiak JP, Kisin ER, Murry AR, Roberts JR, Tyurina YY, et al. Sequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivity. Am J Respir Cell Mol Biol. 2008; 38: 579-590.
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005; 207: 221-231.
- Oyabu T, Myojo T, Morimoto Y, Ogami A, Hirohashi M, Yamamoto M, et al. Biopersistence of inhaled MWCNT in rat lungs in a 4-week well-characterized exposure. Inhal Toxicol. 2011; 23: 784-791.
- Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nature Nanotechnology. 2010; 5: 354-359.