
Research Article
Austin J Biosens & Bioelectron. 2015;1(2): 1010.
The Reaction Kinetics of Glutathione Capped Quantum Dots for the Detection of Hydrogen Peroxide
Imtiaz Ahmad¹, Haife Zhang² and King-Tong Lau¹*
¹Department of Chemistry, Xi’an Jiaotong-Liverpool University, China
²Department of Chemistry, University of Liverpool, UK
*Corresponding author: Kim King Tong Lau, Department of Chemistry, Xi’an Jiaotong-Liverpool University, Suzhou, China
Received: October 13, 2014; Accepted: February 14, 2015; Published: March 31, 2015
Abstract
Glutathione capped cadmium tellurium quantum dot was investigated as the fluorescence probe for the detection of hydrogen peroxide. In this detection regime, we regard QDs as only the signal transducer for the pseudo first-order reaction between hydrogen peroxide and glutathione. In this way the relationship between the rate of QD fluorescence decay and the rate of change in hydrogen peroxide concentration during the reaction were established and experimentally validated. Hence, the rate constants for the reaction were evaluated. The application of kinetic measurement to chemical analysis was presented and the results were comparable to those obtained from normal measurement at equilibrium.
Keywords: Quantum dots; Hydrogen peroxide sensing; Optical sensors; Fluorescence; Reaction kinetics
Introduction
Hydrogen peroxide is an important metabolic by product of various physiochemical and pathological processes that involve molecular oxygen. There are many circumstances where the concentration of hydrogen peroxide needs to be measured. Cellular metabolism process involves reduction of molecular oxygen to produce hydrogen peroxide. High concentration of H2O2 can produce reactive oxygen species, which potentially causes DNA impairment and propagate cancer [1-3]. Enzymes such as oxidases produce hydrogen peroxide as a byproduct, [4] hence it is also used as a principal indicator for the detection of a range of important biomolecules, including glucose, [5] cholesterol [6] and triglyceride [7].
Many different sensing techniques have been used for the detection of H2O2 [2,8]. Among these the fluorescence approach [9,10] offers rapid measurement and excellent sensitivity, and the technique has been reported to be used for single molecule tracking [9,11]. The recent emergence of colloidal semiconductor quantum dots has brought significant advancement in fluorescence based sensing [12,13]. QDs have unique size dependent fluorescence emission with narrow emission peak as a result of the quantum confinement effect. Contrast to organic dyes, QDs offer higher photo stability, high fluorescence quantum yield and they can be excited by broad excitation wavelengths [14]. Further, the physical and chemical properties of QD nanocrystals can be tuned by a selection of capping molecules with specific physical and chemical properties [15] which, among other things, provide the QDs with water solubility, biocompatibility and high quantum yield [16].
Glutathione is a natural antioxidant that regulates the redox state of biological system [17] Owing to its biocompatibility, glutathione has been used as capping agent for QDs for use in bio-imaging and sensing applications [18,19] QDs capped with thiols are sensitive to oxidizing agents such as hydrogen peroxide; hence they can be used as chemical sensor to detect these oxidants. The detection of H2O2 by QDs is attributed to the reaction between the surface bound glutathione molecules and hydrogen peroxide. The surface bound glutathione is oxidized to the corresponding disulphide as the main product; this change in surface characteristics disrupts the electronhole recombination process of the QD, resulting in the fluorescence quenching [12].
Mono-dispersed QDs sample is rather like a polymer, consists not of truly mono dispersed particles but of a range of particle sizes or molecular weights. Although the stoichiometry of the reaction between H2O2 and free glutathione in solution to give the corresponding disulphide is well understood, there are still many uncertainties regarding to using QDs as a reagent for H2O2measurement. Further, the degree of QD surface disruption (i.e. the amount of glutathione reacted) to effect measurable fluorescence change has not been established. Hence the reaction stoichiometry between H2O2 molecules and QD particles is not known. Therefore, it is necessary to establish the relationship between the measured QD fluorescence change and the change in H2O2 concentration to validate the use of QDs for the detection of H2O2.
In this work we have synthesized glutathione capped CdTe quantum dots for the study of the fluorescence degradation of QDs by H2O2. A kinetic model is proposed for a pseudo first order reaction with respect to H2O2; in which the rate of change of QD fluorescence intensity is related to the rate of diminishing H2O2. Experimental data were then used to verify the model and evaluate the rate constants.
Experimental
Chemical s and materials
All the chemicals used were of analytical grade. Tellurium (v) Oxide 99%, citric acid trisodium salt dihydrate 99%, cadmium chloride 99%, sodium borohydride 98%, hydrogen peroxide 30%, glutathione,dipotassium hydrogen phosphate, dihydrogen potassium phosphate and sodium hydroxide were purchased from Acros Chemicals, USA. Milli-Q water (Millipore Co., Billerica, MA, USA) was used for all experiments.
QD Synthesis
Glutathione (GHS) capped cadmium tellurium Quantum Dots (QDs) with red emission was synthesized in-house following reported procedure with modification [20]. In brief, eight mL of 0.04 M cadmium chloride was added to in a round bottomed flask containing trisodium citrate dihydrate (0.2 g), glutathione (0.1 g), TeO2 (0.01 M, 2 mL) in 65 mL of water.NaBH4 (0.1 g) were added with stirring. The mixture was reacted at 90 oC under open-air conditions for a certain period of time. The obtained QDs were precipitated with 1-propanol and the precipitates were separated by centrifugation and were redissolved in50 mM phosphate buffer solution (pH 7.2). The precipitation was repeated three times in order to eliminate the free glutathione ligands from the CdTe QDs colloids.
Characterization of QDs
Fluorescence spectra were obtained with Fluoromax-4 Spectrofluorimeter, Horiba Scientific; uv-vis spectra were obtained with Cary 300 UV-VIS Spectrometer, Agilent Technologies; infrared spectra were obtained with Cary 600 Series FTIP Spectrometer, TEM data were obtained with HR-STEM Transmission Electron Microscope Tecnal G2 F20, FEI Company, USA.
Equilibrium state H2O2 measurement
Buffer solution and hydrogen peroxide solutions were made up in Milli-Q water. QDs solution (0.238 g/L) was made up in 10 mM potassium phosphate buffer adjusted to pH 7.2. 100 mM H2O2 stock solutions were used for analysis. Aliquots of 5, 10, 15, 20, 30, 40, 50 and 60 μL of 100 mM freshly prepared H2O2 stock solution were added into 25 mL QDs solution in a 50 mL conical flask with continues stirring. After each addition of H2O2 the reaction was allowed to proceed for 10 minutes before fluorescence analysis. An excitation wavelength of 420 nmwas used to obtain the all emission spectra.
Kinetic measurement
In brief, a series of H2O2 standards with concentrations of 1.5, 3, 6, 12 and 24 mM were made up in Milli-Q water from a 0.6 M H2O2 stock solution. 10 μL of H2O2 standard was added into a 1 mL cuvette, followed by 1 mL of QDs solution (0.238 g/L). The change of fluorescence intensity at 595 nm was monitored by using an excitation wave length of 420 nm. A sampling rate of 10 data points per second was used and the data were exported to ExcelTM for analysis.
Results and Discussion
Synthesis and characterization of QDs
The one pot synthesis method allows the control of the QDsize simply by monitoring the colour and emission spectrum of the product. Hence, QDs of different sizes were obtained with different reaction time. The uv-vis absorption and emission spectra of the products are presented in Figure 1 & 2 respectively. Table 1 summarized the absorption and emission characteristics of the QDs obtained with different reaction time. The size of the QD products was estimated from the uv-vis absorption spectra using the method described in reference [21].
Reflux time (min)
?max Absorbance (nm)
?max
Emission (nm)
Emission FWHM
(nm)
Calculated Size*
(nm)
15
460
480
55.2
0.87
35
480
505
55.1
1.8
90
495
552
55.3
2.3
105
531
578
55.1
3.1
115
553
602
55.1
3.4
*The QD size was calculated from the equation D = (9.8127 x 107) ?3 - (1.7147 x 103) ?2 + (1.0064) ? - (194.84) described in reference [25].
Table 1: A summary of absorption and emission characteristics of glutathione capped CdTe QDs obtained by refluxing for different time. *The QD size was calculated from the equation D = (9.8127 x 107) ?3 - (1.7147 x 103) ?2 + (1.0064) ? - (194.84) described in reference [25].
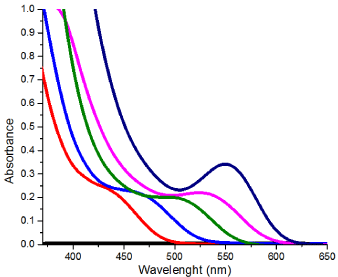
Figure 1: UV-vis spectra of glutathione capped CdTe QDs obtained with
different reaction time.
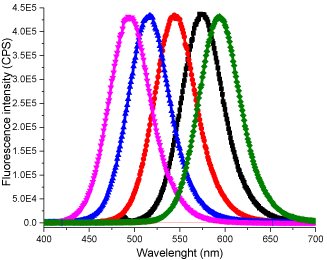
Figure 2: Emission spectra of glutathione capped CdTe QDs obtained with
different reaction time. (Excitation: 420 nm).
The initial product was obtained by heating the reaction mixture at 90 0C for15 minutes, which gave an absorption band with λmax at 460 nm. As the reaction time increased, the resulting QD’s absorption band red shifted gradually to longer wavelengths and the final product gave an absorption band with λmax at 553 nm after heating for 115 minutes (Table 1). The red shift in QD absorption band indicated an increase in band gap was observed and this inferred an increase in QD size due to quantum confinement effect. The fluorescence spectra of the products showed similar trend, giving blue emission with λmax at 480 nm at the beginning and gradually red shifted to 602 nm at the end of reaction. No change in absorbance or fluorescence spectra was detected after the heating time was increased to 180 min. Hence it was assumed that maximum crystal size had been attained with the reaction parameters provided. A TEM image of the QDs with emission at 602 nm is shown in Figure 3. The QDs tend to coagulate on drying as shown by the presence of larger clusters; the size of the single QD identified from the image seems to agree with the calculated result. Presented in Figure 4 are the IR spectra of glutathione capped QD and glutathione itself. The disappearance of the –SH stretching band from the glutathione capped QD compared to pure glutathione proved that the binding of the capping agent to the CdTe crystal is likely to be via the thiol group rather than the acid or amine group, as both of which are still visible from the IR spectrum of the QD.
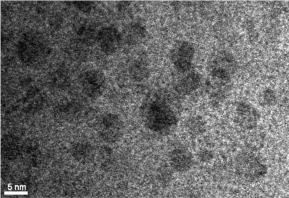
Figure 3: TEM image of glutathione capped CdTe QDs with emission λmax
at 602 nm.
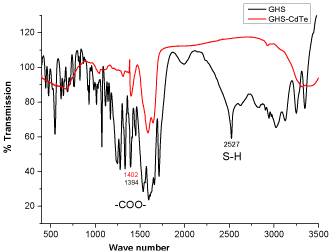
Figure 4: IR spectra of glutathione and glutathione capped CdTe QDs.
The QD product with red emission was selected for the kinetic study because the larger QDsare much easier to reproduce by the synthetic method we employed.
H2O2 measurement at equilibrium
QDs are highly sensitive to physical or chemical alteration of its surface environment. The disruption of its surface bound ligand by chemical reaction will disrupt the electron-hole recombination process to result in emission quenching. H2O2 has been reported to be highly effective in oxidizing glutathione capped QDs to Glutathione disulfide (GHS-SHG) [22,23]. The overall reaction is [24].
H2O2 + 2GSH?GSSG + 2H2O (eq. 1)
Figure 5 presents a set of fluorescence spectra recorded after adding small aliquots (5 to 10 μL) of H2O2 solution into 5 mL of QD solution to give final H2O2 concentrations in the bulk reaction mixture of 20 to 237 μM. The total volume (230 μL) of oxidant added was very small to avoid dilution of the bulk QD solution. After each addition, the mixture was allowed to react for 10 min to reach stable fluorescence intensity before the spectra (3 repeats) were taken. At the end of the calibration, the QD had lost 87% of its original fluorescence intensity, indicating very efficient quenching by H2O2.
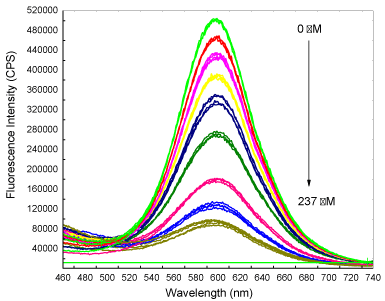
Figure 5: The Fluorescence spectra recorded during H2O2 calibration that
shows a decrease in QD fluorescence intensity with stepwise addition of H2O2
solution. Spectra were recorded in triplicate 10 min after each addition of
H2O2 solution. Excitation wavelength used was 420 nm.
The data (change in intensity) were plotted against H2O2 concentration to yield a linear line (R2 value of 0.9929) with a slope of 1795 cps μM-1 (Figure 6). The Detection Limit (DL) was calculated to be 3 μM by using the standard equation DL = 3.3s / S, where s is the standard deviation of the response and S the slope of the plot. The results show that glutathione capped QDs can provide highly sensitive measurement of hydrogen peroxide that could potentially be useful for in situ measurement of bioprocesses.
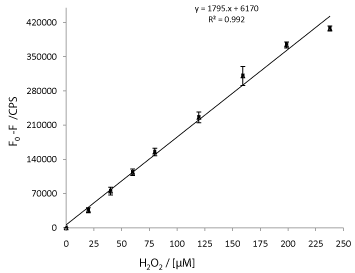
Figure 6: Calibration plot obtained from the data shown in Figure 5.
Kinetics of fluorescence degradation of QDs by H2O2
The fluorescence quenching was a result of the reaction between glutathione capped QDs and hydrogen peroxide; in which the surface bound glutathione was oxidized. The oxidation of surface bound glutathione molecules into the corresponding disulfide re-modified the QD surface which disrupted the electron-hole recombination process to result in the observed fluorescence quenching. Therefore in this detection scheme, the QDs can be seen to function as signal transducer for the chemical reaction between the glutathione and hydrogen peroxide.
There are two important parameters in this reaction that are not well established. Firstly, the loading of glutathione on the QD surface is not determined. Secondly, the degree of surface glutathione oxidation to effect QD fluorescence quenching is not established; which infer that the stoichiometry of hydrogen peroxide and QD reaction is therefore unknown. It has been verified that the measured degradation of fluorescence intensity of glutathione capped QDs is linearly related to the H2O2 concentration. It is therefore possible to make use of the QDs fluorescence degradation as a mean to elucidate the consumption of H2O2 in the reaction mixture.
It was designed that in this detection process, very small amount (10 μL) of dilute H2O2 was added into a 1 mL of strong QD solution (ca. 0.2 mg/mL). The overall concentration of QD and thus glutathione (bound on the surface of QD) are in large excess compared to that of the H2O2;hence the reaction can be regarded as a pseudo first-order reaction with respect to H2O2.
Kinetic model
This model was built on the understanding that the measured degradation of fluorescence intensity of glutathione capped QDs is linearly related to theH2O2 concentration and that the fluorescence intensity of QDs is linearly related to its concentration.
A simple kinetic model can be established with QD being in large excess and the reaction is pseudo first order with respect toH2O2. The rate of fluorescence change can be expressed as:
Rate of change in fluorescence intensity= (1)
Since -
Hence the experimental observed quenching rate is
=
Where k, k’, k’’and K are constants.
Hence,
Therefore, the relationship between fluorescence intensity and H2O2 concentration is established.
For a first order reaction, the observed rate of fluorescence decay is:
Solving (5), we get: F= F0 exp (-kt)(6)
And ln F = ln F0 -k t (7)
Plotting ln Fvs t of eq. (7) gives a linear line with the slopeas the observed fluorescence rate constant k.
Verification of model and evaluation of rate constants
The experimental data for the quenching of QD fluorescence by a series of H2O2 concentrations from 25–200 μMare shown in Figure 7. As expected, the fluorescence quenching appeared to be related to the initial H2O2 concentration. Higher initial H2O2 concentration quenched the fluorescence more rapidly and reached lower fluorescence intensity at the end of the experiment.
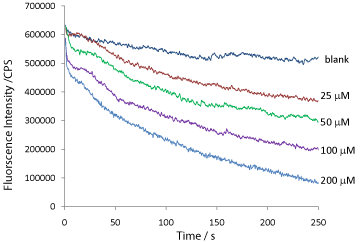
Figure 7: Real time traces of degradation in QD fluorescence intensity by a
series of H2O2 concentrations.
To verify that the kinetic traces obtained follow exponential decay as predicted from a first-order reaction, i.e. eq. (7), [ln F= ln F0- k t], a plot of [ln F]vs [t] is shown in Figure 8. The plot yields a straight line for each of the H2O2 induced fluorescence degradation kinetic traces. From the slopes, the kinetic rate constants k(observed fluorescence decay) were evaluated, and summarized in Table 2.
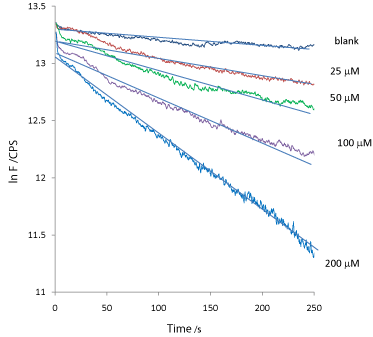
Figure 8: A plot of ln F verses t for the raw kinetic traces data shown in
Figure 7.
[H2O2]/ mM
k / s-1
K / mM-1 s-1
0
0.0006
-
25
0.0019
45.95
50
0.0024
29.02
100
0.0036
21.76
200
0.0071
21.46
Table 2: The experimentally elucidated values of k, the (observed) fluorescence degradation rate constants and K, the rate constant related to hydrogen peroxide concentration.
With known value of k for each starting H2O2concentration, the real relationship between the fluorescence intensity and H2O2concentration can be elucidated from eq.(4), k F = K.[H2O2] at t = 0 or t = t½.
t = 0: kF0 =K.[H2O2]0
t = t½:k F½ = K.[H2O2]½
At the half-life of the reaction i.e. when t = t½,then F = F½ = 1/2F0; and [H2O2]= [H2O2]½ = 1/2[H2O2]0.
For example, the fluorescence degradation trace induced by 200 μMof H2O2; F0 = 604640 cps and k = 0.0071cps. s-1.
Hence,
K = k F0/ ([H2O2]0) = (0.0071cps. s-1) (604640cps) / (200μM) = 21.46 M-1 s-1
Similarly, all K values for each of the starting hydrogen peroxide concentration were calculated and shown in Table 2.
Verification of the order of reaction
The rate constant can be used to evaluate the order of reaction:
Rate of reaction R= k. [H2O2]x.[glutathione]y(8)
From eq. (4): k F = K.[H2O2]
⇒[H2O2] = (k / K) F(9)
Substituting (9) into (8)
Rate of reaction R = (kx+1) / K. [F]x.[glutathione]y(10)
ln (R)= (x+1) ln k – ln K + x ln F + y ln [glutathione] (11)
ln (R) = x ln F + K’(12)
Where K’ = (x+1) ln k – ln K + y ln [glutathione].
From eq.(12), by plotting ln (R) vs. ln F, the order of reaction can be revealed. Figure 9 is a plot obtained with fluorescence decay data induced by 200 μM of H2O2 at time t = 5, 10, 15 and 20 s. The Rate at time t was calculated by Rt= k.Ft.
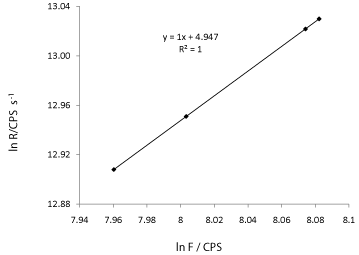
Figure 9: A plot of ln R verses ln F. The slope of 1 indicates a first order
reaction.
The linear plot shown in Figure 9 gives a slope of 1 which indicates that the reaction is pseudo first- order with respect to H2O2.
Kinetic based chemical analysis
The usual mode of chemical measurement mainly relies on performing standard calibration at (pseudo) equilibrium in a timeframe of tens of seconds to tens of minutes. Similar procedure is used for the actual analysis of unknown sample. This method is only practical for reactions that reach equilibrium rapidly to avoid long analysis time, e.g. amperometric measurement, and for reactions that reach completion rapidly, e.g. enzymatic reactions or for reactions that are provided with a well-engineered environment to allow rapid reaction completion e.g. micro fluidics.
In many cases, it is essential to accurately control the time at which the data are taken or it invariably will introduce error into the analysis. For example, in the present study, it is feasible to use the pseudo-equilibrium data to perform calibration as shown in Figure 10. In this plot, the fluorescence intensity data from a specific time instance (100s) for all fluorescence degradation traces was selected for the calibration plot. An excellent straight line with R² = 0.9985was obtained. However, it is easy to imagine that if a onepoint measurement is performed instead of a kinetic trace, the error associated with the time at which the data is collected could be quite large. To reduce such error, it is necessary to increase the analysis time to well beyond 3 minutes, (10 minutes, in the case of data shown in Figure 6) which is rather slow.
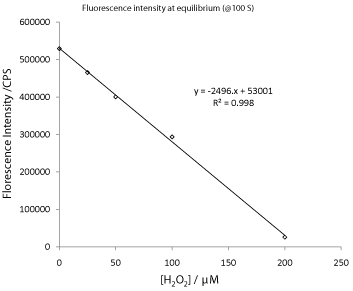
Figure 10: A plot of fluorescence intensity verses hydrogen peroxide
concentration at t = 100 s.
Figure 11 presents the use of kinetic data for quantitative chemical analysis. For better comparison, -k shown in Table 2 were plotted against H2O2concentrationwith base-line corrected to zero. A linear line was obtained with a slope of 3 x 10-5s-1 μM-1. This result suggests that kinetic data has significant analytical value and benefit. The measurement of pseudo-equilibrium data can be prone to error unless extreme care and precautions are taken for reagent mixing and data collection. Whereas the rate constant k is determined by using data collected within a specific time frame rather than a discrete instance of time, it is hence a more robust method of analysis. However, there are drawbacks in using kinetic method for analysis. It is necessary to control the temperature, to reduce sample volume to shorten the analysis time and the mixing has to be instantaneous.
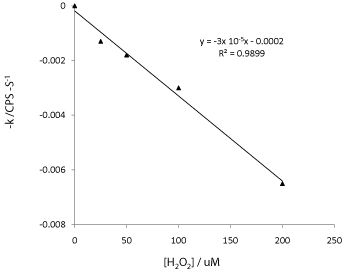
Figure 11: A plot of fluorescence degradation rate constant k verses
hydrogen peroxide concentration.
Conclusion
The use of glutathione capped QDs as fluorescence probe has been validated using kinetic approach. A kinetic model to describe the relationship between the changes in observed fluorescence intensity with the decrease in hydrogen peroxide concentration has been established and validated. Hence the rate constants for the reactions were determined. It is suggested that the kinetic rate constant could be used for qualitative analysis and the results is comparable to those obtained from equilibrium approach.
Acknowledgement
The authors would like to thank XJTLU postgraduate Research Fund for financial support.
References
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005; 17: 183-189.
- Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010; 29: 539-549.
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004; 266: 37-56.
- Ron Gill, Lily Bahshi, Ronit Freeman, Itamar Willner. Angewandte Chemie. 2008; 120: 1700-1703.
- Rahman MM, Ahammad AJ, Jin JH, Ahn SJ, Lee JJ. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors (Basel). 2010; 10: 4855-4886.
- Zhang M, Yuan R, Chai Y, Wang C, Wu X. Cerium oxide-graphene as the matrix for cholesterol sensor. Anal Biochem. 2013; 436: 69-74.
- Minakshi, Pundir CS. Sensors and Actuators B: Chemical. 2008; 133: 251-255.
- Bo X, Ndamanisha JC, Bai J, Guo L. Nonenzymatic amperometric sensor of hydrogen peroxide and glucose based on Pt nanoparticles/ordered mesoporous carbon nanocomposite. Talanta. 2010; 82: 85-91.
- de Silva AP, Gunaratne HQ, Gunnlaugsson T, Huxley AJ, McCoy CP, Rademacher JT, et al. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem Rev. 1997; 97: 1515-1566.
- Brooks Shera E, Seitzinger NK, Davis LM, Keller RA, Soper SA. Chemical Physics Letters. 1990; 174: 553-557.
- Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005; 20: 2555-2565.
- Frigerio C, Ribeiro DS, Rodrigues SS, Abreu VL, Barbosa JA, Prior JA, et al. Application of quantum dots as analytical tools in automated chemical analysis: a review. Anal Chim Acta. 2012; 735: 9-22.
- Jian-Hao Wang, Yong-Qiang Li, Hai-Li Zhang, Hai-Qiao Wang, Song Lin, Jun Chen, et al. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010; 364: 82-86.
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008; 5: 763-775.
- Mark Green. Journal of Materials Chemistry. 2010; 20: 5797-5809.
- Yao-hai Zhang, Hua-shan Zhang, Ming Ma, Xiao-feng Guo, Hong Wang. Applied Surface Science. 2009; 255: 4747-4753.
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983; 52: 711-760.
- Silva FO, Carvalho MS, Mendonça R, Macedo WA, Balzuweit K, Reiss P, et al. Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res Lett. 2012; 7: 536.
- Yuan J, Guo W, Yin J, Wang E. Glutathione-capped CdTe quantum dots for the sensitive detection of glucose. Talanta. 2009; 77: 1858-1863.
- Wang Y, Liu S. J. Chil. Chem. Soc. 2012; 57: 1109-1112.
- Finley JW, Wheeler EL, Witt SC. Oxidation of glutathione by hydrogen peroxide and other oxidizing agents. J Agric Food Chem. 1981; 29: 404-407.
- Abedinzadeh Z, Gardes-Albert M, Ferradini C. Canadian journal of chemistry. 1989; 67: 1247-1255.
- Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999; 27: 322-328.
- Wang Z, Li J, Liu B, Hu J, Yao X, Li J. Chemiluminescence of CdTe nanocrystals induced by direct chemical oxidation and its size-dependent and surfactant-sensitized effect. J Phys Chem B. 2005; 109: 23304-23311.
- Yu WW, Qu L, Guo W, Peng X. Chem. Mater, 2003; 15: 2854-2860.