Editorial
Austin J Biotechnol Bioeng. 2014;1(1): 2.
Neat Mucoadhesive Polymers
Regina Kelmansky and Boaz Mizrahi*
Department of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Israel
*Corresponding author: Mizrahi Boaz, Department of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Technion city, Haifa 32000, Israel.
Received: May 27, 2014; Accepted: June 30, 2014; Published: July 02, 2014
Editorial
Designing mucosal-adhesive (mucoadhesive) materials are of great importance as an advanced tool for wound healing and covering, bleeding control, drug delivery and other medical applications [1]. Mucoadhesive polymers can be used to treat numerous diseases. For example: Canker sores heal faster with mucoadhesive tablets [2], mucoadhesive gel can be used to treat Periodontitis [3] or vaginal infections [4], while mucoadhesive eye drops for Glaucoma treatment [5]. Although various muco-adhesive formulations have been developed, tradeoffs between adhesive properties and ease of use occasionally limit their clinical use. Solid formulations such as tablets and films adhere very strongly to the mucosal tissue with a very long time of residency [6], however, they are often considered too rigid and lack flexibility [7]. In contrast, liquid formulations are very well tolerated by the patients, but adhere weakly to tissue due to their weak structural integrity. Thus, development of a new biomaterial that would combine the advantages of solid and of liquid formulations is desired: Convenient liquid formulation that solidifies in-situ after administration to yield a strong and long lasting adherence.
Currently used mucoadhesive polymers are either from a synthetic or a natural origin. The synthetic group mostly consists of polyacrylic acid-based polymers (e.g. polyacrylate) and cellulose derivatives (e.g. hydroxypropyl cellulose). Common examples for semi-natural polymers are chitosan and various gums like alginate and pectin. All of the above mentioned and other existing materials, are solids in their basic form that require a relatively high solvent content in order to serve as hydrogel forming agents [7].
High solvent (e.g. water) content is a major reason limiting the adhesive properties of currently used mucoadhesives, particularly when combined with material's porosity [8]. Therefore, a neat (without solvent), yet liquid prepolymer system may overcome shortcomings in mechanical properties. Examples of such systems are multi armed polyethylene glycol (2000 Da) modified with various functional groups [9,10]. The advantages of multi armed, neat systems include: (a) they are liquid at room temperature [11] and, therefore, can be applied without the need of solvent; (b) although these polymers have low viscosity at room temperature, they can rapidly harden when crosslinked; (c) these polymers possess a higher number of potentially reactive end groups per molecule compared to linear polymers of similar molecular weight; (d) it is relatively easy to control the viscosity of these materials by varying the molecular weight and/or crosslinking degree; and (e) they have low immunogenicity and toxicity [12,13].
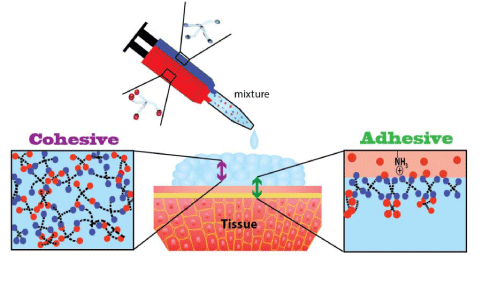
Adhesiveness of the cross-linked system can be evaluated with rotational rheometry [14] and tensiometry [15,16], or other methods depending on the specific situation [17,18]. The criteria for success in many of these tests need to be both analyzed and discussed with selected clinicians who use or would potentially use these adhesives. As with any adhesive system, viscosity measurements are critical to characterize structural changes over time. Mixtures of two complementary different pre-polymers (e.g. PEG-amine with PEG-aldehyde) commonly display rheological synergism, in that the viscoelastic properties of the polymer blends are dramatically more solid-like than those of the constituent polymers. A relevant advantage of these formulations is that they can be easier to inject than less viscous single-polymer solutions. Adherence of mucoadhesive polymers to tissue is dependent upon obtaining close contact between the tissue and biomaterial (adhesive strength), and on the integrity of the mucoadhesive material (cohesive strength of the material) (Figure 1). A covalent bond between the adhesive and the tissue (e.g. by the formation of imine bonds from polymer's carboxylic acid and tissue's amine) yields a stronger contact than weaker bonds (e.g. ionic bonds, Van der Waals forces, hydrophobic interaction, etc.). Additionally, the attachment strength is highly dependent on the amount of the reactive groups presented on the biomaterial. Measurements of gluing times, strength, and effectiveness over time are therefore required as well as determining the failure point which is critical for assessing the success of the materials, depending on the clinical use for which they will be designed for [19].
Figure 1: Illustration of the mucoadhesion system; mixture of the two neat components leads to the formation of cohesion bonds inherent within the material, and adhesion bonds - of the same chemistry - between the material and the tissue.
Therapy of mucous surfaces often requires the delivery of medical substance. The ability to maintain a delivery system at a particular location for an extended period of time has many advantages for both local disease treatments as well as for systemic drug bioavailability. The relatively high water content of currently used mucoadhesives often leads to rapid drug release kinetics or to incompletion of drug release due to possible evacuation from the site. Regardless of the desired release pattern, drugs with poor water solubility may be hard to contain in an aqueous based hydrogel. The absence of solvent in the neat systems may allow the dissolution of water-soluble as well as of poorly water-soluble drugs [10]. Moreover, neat systems demonstrate a more sustained release pattern compared to typical hydrogels, which can be attributed to the relatively high polymer mass per unit volume [20].
In conclusion, designing a neat liquid mucoadhesive formulation that solidifies in situ could potentially revolutionize wound healing and regenerative medicine as known today. These biomaterials may also have far-reaching applications in other related fields such as tissue adhesives and tissue engineering. We expect that these polymers, which in the past have been rarely studied, will form the basis for the systematic investigation of neat systems and will also become a powerful tool for studying the therapeutic role of bio-adhesive materials. Conjugation of these polymers with functional groups is expected to produce a large number of potential materials with a range of interesting and desired properties.
Acknowledgment
The research leading to these results has received funding from the European Union's-Seventh Framework Programme (FP7/2007-2013]) under grant agreement n° 631280 - MC-Neat Bio-Adhesives.
References
- Artzi N, Edelman ER. Aldehyde-Amine Chemistry Enables Tissue Adhesive Materials to Respond to Physiologic Variation and Pathologic States. Isr J Chem. 2013; 53: 748-755.
- Artzi N, Edelman ER. Aldehyde-Amine Chemistry Enables Tissue Adhesive Materials to Respond to Physiologic Variation and Pathologic States. Isr J Chem. 2013; 53: 748-755.
- Bansal K, Rawat MK, Jain A, Rajput A, Chaturvedi TP, Singh S. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech. 2009; 10: 716-723.
- Acartürk F. Mucoadhesive vaginal drug delivery systems. Recent Pat Drug Deliv Formul. 2009; 3: 193-205.
- Mazor Z, Ticho U, Rehany U, Rose L. Piloplex, a new long-acting pilocarpine polymer salt. B: Comparative study of the visual effects of pilocarpine and Piloplex eye drops. Br J Ophthalmol. 1979; 63: 48-51.
- Duchêne D, Ponchel G. Principle and investigation of the bioadhesion mechanism of solid dosage forms. Biomaterials. 1992; 13: 709-714.
- Mizrahi B, Domb AJ. Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent Pat Drug Deliv Formul. 2008; 2: 108-119.
- Calvert P. Hydrogels for Soft Machines. Adv Mater. 2009; 21: 743-756.
- Mizrahi B, Khoo X, Chiang HH, Sher KJ, Feldman RG, Lee JJ, et al. Long-lasting antifouling coating from multi-armed polymer. Langmuir. 2013; 29: 10087-10094.
- Mizrahi B, Shankarappa SA, Hickey JM, Dohlman JC, Timko BP, Whitehead KA, et al. A Stiff Injectable Biodegradable Elastomer. Adv Funct Mater. 2013; 23: 1527-1533.
- Younes HM, Bravo-Grimaldo E, Amsden BG. Synthesis, characterization and in vitro degradation of a biodegradable elastomer. Biomaterials. 2004; 25: 5261-5269.
- Burke SA, Ritter-Jones M, Lee BP, Messersmith PB. Thermal gelation and tissue adhesion of biomimetic hydrogels. Biomed Mater. 2007; 2: 203-210.
- Figueiredo VM. A five-patient prospective pilot study of a polycaprolactone based dermal filler for hand rejuvenation. J Cosmet Dermatol. 2013; 12: 73-77.
- Madsen F, Eberth K, Smart JD. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J Control Release. 1998; 50: 167-178.
- Tamburic S, Craig DQM. A comparison of different in vitro methods for measuring mucoadhesive performance. European Journal of Pharmaceutics and Biopharmaceutics. 1997; 44: 159-167.
- Bundy K, Schlegel U, Rahn B, Geret V, Perren S. An improved peel test method for measurement of adhesion to biomaterials. J Mater Sci Mater Med. 2000; 11: 517-521.
- Nakayama Y, Matsuda T. Photocurable surgical tissue adhesive glues composed of photoreactive gelatin and poly (ethylene glycol) diacrylate. J Biomed Mater Res. 1999; 48: 511-521.
- Carbon RT, Baar S, Kriegelstein S, Huemmer HP, Baar K, Simon SI, et al. Evaluating the in vitro adhesive strength of biomaterials. Biosimulator for selective leak closure. Biomaterials. 2003; 24: 1469-1475.
- Korkmaz Y, Gurgan S, Firat E, Nathanson D. Shear bond strength of three different nano-restorative materials to dentin. Oper Dent. 2010; 35: 50-57.
- Zhang XZ, Wu DQ, Chu CC. Synthesis, characterization and controlled drug release of thermosensitive IPN-PNIPAAm hydrogels. Biomaterials. 2004; 25: 3793-3805.