Department of Biotechnology, SGB Amravati University, India
*Corresponding author: Priti Paralikar, Department of Biotechnology, SGB Amravati University, Amravati 444602, Maharashtra, India.
Received: October 22, 2014; Accepted: December 05, 2014; Published: December 08, 2014
Silver nanoparticles have a huge share in nanotechnology, which offers numerous benefits, including ecofriendliness and compatibility for biomedical applications. Here, we report the synthesis of silver nanoparticles due to the reduction of silver nitrate by an aqueous leaf extract of Epiphyllum oxypetalum. The synthesized silver nanoparticles were characterized by UV-Visible spectroscopy, NTA analysis, FT-IR analysis and Zeta potential. The results showed that silver nanoparticles thus synthesized have an average particle size of 86 nm. The antibacterial activity of the synthesized silver nanoparticles alone and in combination with commercial antibiotics was tested against Propionibacterium acne, Pseudomonas aeruginosa and Klebsiella pneumoniae. From this study, it can be concluded that efficiency of silver nanoparticles increased when accessed in combination with antibiotics against test organisms. Due to pronounced antibacterial activity, silver nanoparticles synthesized by leaf extract of E. oxypetalum can act as effective antibacterial agent for various biomedical applications.
Keywords: Antibiotics; Epiphyllum oxypetalum; Silver nanoparticles
Nanotechnology is rapidly growing multidisciplinary branch of science with enormous applications in various fields due to involvement of its building blocks such as nanoparticles. Nanoparticles are significantly different from larger materials due to their improved properties based on specific characteristics such as size, distribution and morphology [1]. Nanomaterials are creating progress due to their chemical, magnetic, optical properties than bulk materials [2].
Metal nanoparticles are more effective because of high surface to volume ratio. Therefore, in case of silver nanoparticles (AgNPs), large proportions of silver atoms are in direct contact with their environment [3]. Among all metal nanoparticles, AgNPs have great importance due to their application in various fields such as nanomedicine, antimicrobial agent [4], in ground water purification [5], AgNPs are also used for the preparation of surgical masks [6]. Duran et al. [7] developed AgNPs impregnated wound dressings and textile fabrics, which can be used for burnt patients. The unique properties of metal nanoparticles, also extend their applications in catalysis, biological tagging, drug delivery, diagnostics, imaging, sensing, gene delivery, artificial implants and tissue engineering [8].
Researchers reported that AgNPs can be effectively used against multi-drug-resistant bacteria [9,10] because of their large surface area and small size makes them easy to interact with substances and increases their antibacterial efficacy. AgNPs can be the new generation of antimicrobials with broad-spectrum activity [11,12] and it can be used in many antimicrobial preparations [13]. Biological methods for nanoparticle synthesis have some advantages over chemical and physical methods because these methods involve the use of toxic chemicals and also sometimes reactions takes place at very high temperature [14,15]. However, biological methods for synthesis are cost effective and less hazardous. The use of plants for synthesis of nanoparticles could be advantages over other microorganisms because it eliminates the process of maintaining of culturing.
Various plant systems have been used for the biosynthesis of silver nanoparticles, which includes Azadirachta indica [16,17], Carica papaya [18], Murraya koenigii [19,20], Ananas comosus [21], Annona reticulate [22], Foeniculum vulgare [23], Catharanthus roseus [24], Securinega leucopyrus [25], Cardiospermum halicacabum [26], Lawsonia inermis [27], Camellia sinensis [28], Mollugo mudicaulis [29], Opuntia ficus-indica [30], Lycopersicon esculentum [31] and Musa balbisiana [32].
In the present study, we have used Epiphyllum oxypetalum (vernacular name -Brahma Kamal) for the synthesis of AgNPs. This plant is very unique and interesting, it belongs to Cactaceae family. It is commonly known as Night-blooming Cereus, Dutchman’s Pipe, Queen of the Night. It is known as Brahma Kamal in India and treated as a sacred plant. E. oxypetalum was the most commonly grown of the Epiphyllum species. It is known as orchid cactus because it has orchid- like beauty and resemble with cactus in habit. The generic name Epiphyllum refers to flower born on leaf- like phylloclade and species oxypetalum refers to acute petals of this species. Upendra et al. [33] reported antibacterial activity of solvent extract of leaves of E. oxypetalum amoung them maximum activity was exhibited by acetone and petroleum ether leaf extract aginst selected test organisms.
Antibacterial activity of AgNPs synthesized by leaf broth of Gliricidia sepium was reported by Raut et al. [34]. Gaikwad et al. [35] demonstrated the antiviral activity of AgNPs synthesized from fungi isolated from infected leaves of plants. Similarly, antibacterial and antifungal activity of AgNPs was reported by Devi et al. [36]. In their study, the activity of AgNPs synthesized from Hypnea muciformis which is marine macro red algae was evaluated. Moreover, Jena et al. [37] reported the antimicrobial potential of AgNPs synthesized using diatoms Amphora sp.
In the present study, E. oxypetalum was used for synthesis of silver nanoparticles, which were evaluated against P. acne, P. aeruginosa and K. pneumoniae and also in combination of commercially available vancomycin to study synergistic activity.
The young healthy leaves of E. oxypetalum were collected from local garden of Amravati (Maharashtra), India. AgNO3 was purchased from Hi-Media® Pvt. Ltd. Mumbai, Maharashtra.
Propionibacterium acne (MTCC 1951), Pseudomonas aeruginosa (MTCC 4676), Klebsiella pneumonia (MTCC 4030) were procured from microbial type culture collection center, IMTECH, Chandigarh, India and used as test bacteria.
The leaves of E. oxypetalum were washed 2-3 times with tap water followed by sterilized double distilled water. Ten gm of leaves were weighted and cut into fine pieces and crushed with 100 ml of sterilized doubled distilled water using blender. Crude extract was filtered through muslin cloth 2 times and then centrifuge at 4000 rpm for 25 minutes to obtain clear leaf extract which was later used for synthesis.
Ten-ml of above prepared extract was challenged against 90 ml of 1mM silver nitrate (AgNO3) solution and incubated at room temperature. The synthesis of silver nanoparticles was indicated by development of its characteristic brown colour. The purified silver nanoparticles were obtained on treatment of acetone with silver colloids leads to the precipitation of silver nanoparticles The obtained reaction mixture was subjected for further characterization.
Visual observation: The colour change in reaction mixture after treatment of plant extract with 1mM AgNO3 was recorded through visual observation.
UV-Visible analysis: The UV spectra of synthesized nanoparticles were recorded using Shimadzu UV-1700 instrument at the range of wavelength 200-800 nm.
Nanoparticles tracking analysis (NTA): Synthesized nanoparticles were characterized by NTA using LM-20 (NanoSight Ltd. UK) to find out average particle size. Liquid sample of AgNPs at the concentration of 107-109 /ml were introduced into scattering cell through, which laser beam was passed. The particles in suspension of path of this beam can easily visualized by microscope objective. The Brownian motion of nanoparticles within the path of laser beam was detected by Charge Coupled Device (CCD) camera. The generated videos and images were analyzed for size distribution of nanoparticles.
Zeta potential: Zeta potential of synthesized nanoparticles was analyzed to determine the charge present on the particles. Zeta potential was measured by Zetasizer nano series (Malvern) instrument in the range between 0.1-1000 μm.
Fourier transform infrared spectroscopy (FTIR): The colloidal solution of AgNPs, were centrifuged at 20,000 rpm for 20 min and then the pellet was collected. The collected pellet was resuspended in supernatant of AgNPs solution. Fourier transform-infrared spectroscopy (FT-IR) used for analysis AgNPs of by scanning the spectrum in the range 550–4000 cm-1 at a resolution of 16 cm-1 was carried out.
The antibacterial activities of AgNPs were evaluated against P. acne, P. aeruginosa and K. pneumoniae. The standard antibiotic discs of vancomycin were purchased from Hi-Media® Pvt. Ltd Mumbai, Maharashtra, India. The overnight grown bacterial cultures having CFU 105-106/ml were used to access the activity. The antibacterial activity was performed by Kirby-Bauer disc diffusion method. The assay was performed on Muller Hinton agar plates. The inoculum was spread over agar plates using sterile Hi-media cotton swab. The activities of nanoparticles were evaluated in combination with antibiotics by impregnating 20 μl of nanoparticles solution in antibiotic disc. Similarly, with AgNPs and aqueous plant extract control on respective discs which were incubated at 37°C for 24 hrs followed by measurement of zone of inhibition. The assay was carried out in triplicate.
The increase in fold area was assessed by calculating the mean surface area of the inhibition zone of each tested antibiotic. The fold increase area of antibiotic was calculated against different test organisms by the equation (b2 - a2) / a2, where ‘a’ and ‘b’ are zone of inhibitions for antibiotic (A) and antibiotic + Ag-NPs (B), respectively.
In the present study, leaf extract of E. oxypetalum (Brahma Kamal) was successfully used for the synthesis of AgNPs. After treatment of aqueous plant leaf extract with 1mM AgNO3 colour change was observed in the reaction mixture from light -green to dark- brown (Figure 1). It takes about 5-10 minutes to complete the reaction with the 30 sec exposure to sunlight. The change in colour indicated the formation of AgNPs which occurs due to excitation of surface plasma resonance in metal nanoparticles [3,9].
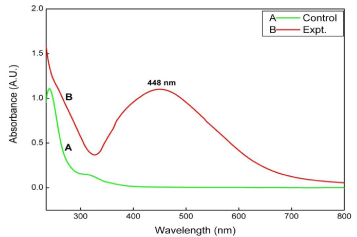
Absorption measurement was carried out using UV-Visible spectrophotometer at a resolution of 1 nm. The UV- Visible spectra of synthesized AgNPs showed absorption peak at 448 nm which is specific for AgNPs. These results showed resemblance with many previous studies. Jain et al. [38] showed the absorbance peak at 450 nm for AgNPs synthesized using spore crystal of Bacillus thurenginesis. The results obtained ensure the existence of AgNPs in the solution. Dar et al. [39] demonstrated that AgNPs showed sharp peak around 440 nm. The UV- Visible spectra of AgNPs was shown in Figure 2.
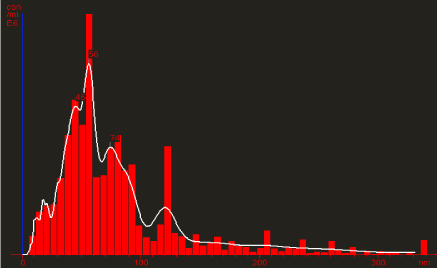
Further, nanoparticles thus synthesized were characterized by Nanoparticles Tracking and Analysis (NTA) device (Nanosight LM- 20). The NTA technique looks at individual nano-particles and sizes them on a particle –by- particle basis. Inherent weakness in techniques such as DLS (Dynamic Light Scattering), or PCS (Photo-correlation Spectroscopy), which produce an average particle size, are overcome in NTA. NTA can also go to much smaller particle sizes (down to 10 nm) than light obscuration techniques, which is an added advantage of this technique. The distribution of particle size to concentration of AgNPs was shown in Figure 3. The analysis confirm the synthesis of polydisperse AgNPs having size ranging between30-86 nm. However, the total concentration of synthesized AgNPs was found to be 2.10 particles/frame and 0.08 particles/ml.
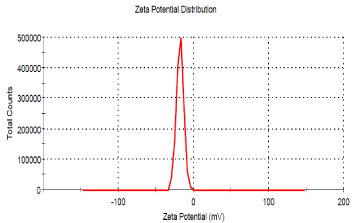
Zeta potential analysis was also carried out to detect the surface charges acquired by AgNPs. The zeta potential of synthesized nanoparticles was found to be -18 mV which indicates that particles were relatively stable. Gurunathan et al. [40] reported that negative zeta potential value of the particles might be due to adsorption of OH-ions on it. OH- ion helps in preventing the aggregation and maintains the smaller size of AgNPs. It was also reported that greater the zeta potential, greater the stability of nanoparticles in colloidal state [35]. The zeta potential distribution graph was shown in Figure 4.
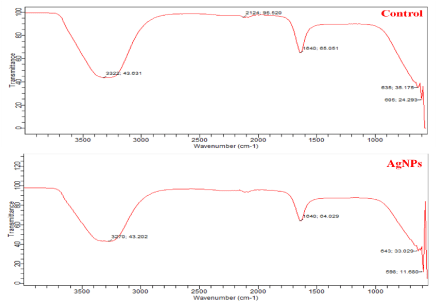
Later, FT-IR analysis of AgNPs was carried out to identify the possible biomolecules responsible for synthesis and stabilization of AgNPs. The FTIR spectra showed the presence of peaks at 3270 cm-1, 1640 cm-1, 643 cm-1 and 598 cm-1 in experimental and 3322 cm-1, 2124 cm-1, 1640 cm-1, 635 cm-1 and 605 cm-1 in control spectrum (Figure 5). In experimental spectrum the intense band observed at 3270 cm-1 is the characteristic of the hydroxyl functional group in alcohol and phenol compounds [41]. The band at 2124 cm-1 may be assigned with –C-H stretching [42] in control spectrum. The peak at 1640 cm-1 in both the spectrum is associated with the amide band leads primarily to bending vibrations of the N—H bond [43]. So from overall observation confirms the presence of protein in sample which coats the silver nanoparticles as a capping protein and this stabilizes the silver nanoparticles.
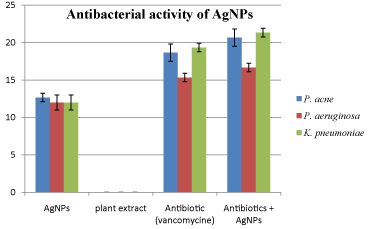
In vitro antibacterial activity of synthesized AgNPs from E. oxypetalum alone and in combination with commercially available antibiotic (vancomycin) was evaluated against P. acne, P. aeruginosa and K. pneumoniae. The results clearly indicate that synthesized AgNPs exhibit significant antibacterial activity against all the test organisms and it was calculated from the zone of inhibition. Thus, synthesized AgNPs were found to have almost similar activity against all the bacteria. In case of P. acne it showed somewhat higher activity showing zone of inhibition or 12.6 mm. However, it showed similar zone of inhibition for P. aeruginosa (12 mm) and K. pneumoniae (12 mm) On the other hand, no zone of inhibition was observed in case of plant extract. Similarly, the activity of AgNPs was also studied in combination with vancomycin. The observations revealed that the activity of commercial antibiotics (vancomycin) was increased when used in combination and it was calculated from increase in fold area. It was found the combination of AgNPs and vancomycin showed maximum activity against P. acne (increase in fold area= 0.184) followed by K. pneumoniae (increase in fold area= 0.178) and P. aeruginosa (increase in fold area= 0.153). Details are shown in Table 1. However, the graphical distribution of antibacterial activity of AgNPs was shown in Figure 6.
This is the first report for the synthesis of AgNPs using leaf extract of E. oxypetalum and it is found to be easy, fast and green approach. E. oxypetalum is capable of producing AgNPs extracellular and these AgNPs are quite stable in solution due to capping likely by the proteins present in the extract. Further, the synthesized AgNPs showed significant bactericidal activity against Gram positive and Gram negative bacteria. This method is an eco-friendly and cost-effective way of synthesis of silver nanoparticles under laboratory as well as room conditions. Due to pronounced bactericidal activity, AgNPs synthesized by E. oxypetalum, can be used as effective antibacterial agent for various biomedical applications.
I am thankful to Prof. M. K. Rai, Dr. A. K. Gade, Dr. A. P. Ingle and S. C. Gaikwad for their guidance and support; and to Funds for Infrastructure Science and Technology (FIST) for providing computer facility, Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati.
Zone of inhibition (mm) against P. acne, P. aeruginosa and K. pneumoniae of AgNPs, Antibiotic and AgNPs in combination with antibiotic at concentration of 20μl/disc.
Test Organism |
Zone of Inhibition in mm |
Increase in fold area |
|||
AgNPs |
Plant Extract |
Antibiotic (vancomycin) |
Antibiotic + AgNPs |
||
P. acne |
12.66±0.57 |
0 |
18.66±1.15 |
20.66±1.154 |
0.184 |
P. aeruginosa |
12.00±1 |
0 |
15.33±0.57 |
16.66±0.57 |
0.153 |
K. pneumoniae |
12.00±1 |
0 |
19.33±0.57 |
21.33±0.57 |
0.178 |
Control (left) and silver (right) nanoparticles synthesized from Epiphyllum oxypetalum.

UV-Visible spectra of synthesized silver nanoparticles from Epiphyllum oxypetalum.
Particle size distribution of synthesized silver nanoparticles using Epiphyllum oxypetalum.
The zeta potential distribution graph of silver nanoparticles synthesized using Epiphyllum oxypetalum.
FT-IR spectrum of leaves extract (control) and synthesized silver nanoparticles.
The graphical representation of antibacterial activity of silver nanoparticles.