
Research Article
Austin J Biotechnol Bioeng. 2015;2(1): 1037.
Dicyandiamide Reduces Nitrous Oxide Emission in Paddy Soils
Wu DL1, Zheng SS1, Mahmood Q2, Li JY1, Tian GM1*, Li H1 and Li YX1
1Department of Environmental Engineering, College of Natural Resources and Environmental Science, Zhejiang University, China
2Department of Environmental Sciences, COMSATS Institute of Information Technology, Pakistan
*Corresponding author: Qaisar Mahmood, Department of Environmental Sciences, COMSATS, Institute of Information Technology, Pakistan
Tian GM, Department of Environmental Engineering, ollege of Natural Resources and Environmental Science, Zhejiang University, China
Received: December 12, 2014; Accepted: March 09, 2015; Published: March 11, 2015
Abstract
The effects of various Dicyandiamide (DCD) concentrations and variations of watering on the mechanism of nitrogen transformations and Nitrous Oxide (N2O) emission in paddy soils were investigated. The application of low concentration of DCD (0.5% of urea) delayed the N2O emission peak for 3 days while high DCD level (2% of urea) almost completely inhibited the N2O emission. Under 95% and 110% WSPF conditions, soil nitrite concentration increased and reached the peak on the 7th day with 0.5% DCD application same as in the control, while it was very low all the time in the 2% DCD treatment. Under 60% WSPF, nitrite in the soil pool decreased for all DCD treatments. Strong inhibitory effects of DCD were noted on nitrosobacterial populations without any obvious influence on soil nitrobacteria and denitrifying bacteria. At higher DCD applied level under fully moist conditions (110% Water Filled Pore Spaces (WSPF)), nitrite and nitrate concentrations kept very low throughout indicating that initial step of nitrification was completely inhibited. It was concluded that 2% DCD may be applied under fully moist conditions to increase in rice yield and to avoid release of N2O.
Keywords: DCD; Nitrous oxide; Nitrogen transformation; Water conditions
Introduction
More orthodox style of rice cultivation in China involves continuous flooding of paddy fields for about a month after application of basal fertilizer, then exposed to aeration for a week or so named as Midseason Aeration (MSA) which are followed by intermittent irrigations. It was proposed that nitrification inhibitor may be inefficient during continuous flooding, as stagnant water creates anaerobic conditions, thus automatically suppressing the nitrification [1,2]. Rapid drainage and drying– wetting alternation may render the upper soil layers aerobic in rice fields. Considerable amount of N2O is produced via nitrification as well as denitrification and lost to atmosphere. Nitrous Oxide (N2O) is produced as a result of natural denitrification in rice fields which contributes to global warming [3]. It is 298 times more intoxicating as than CO2 and its concentration in the atmospheric is rising at the rate of approximately 0.26% year-1 and reached 319 ppb (10-9 mol mol-1) in 2005 [4]. The global warming potential of N2O is 320-340 times higher than that of Carbon Dioxide (CO2) when calculated for a time horizon of 100 years [5].
Approximately 80% of total atmospheric N2O is released from agricultural soils caused by nitrogen fertilizers which are the most important anthropogenic sources of N2O [6]. Apart from environmental concern, such losses lead to lower crop productivity. An approach of minimizing the nitrate leaching and denitrification from soil and improving nitrogen use efficiency is to use a nitrification inhibitor [7] which delays the NO3 - production from NH4 +. Urease and nitrification inhibitors weaken the urea hydrolysis to NH4 +–N and inhibit the nitrification process of NH4 +–N, respectively, thus inhibiting the N2O formation. Traditionally, urease and nitrification inhibitors are applied into soil along basal fertilizers prior to rice transplantation. Hydroquinone (HQ) - the Urease inhibitor and Dicyandiamide (DCD) as nitrification inhibitor are currently being used in this field. DCD alone or in combination with HQ, could substantially reduce CH4 and N2O emissions during rice growth season [8,9] and effectively regulated the behavior of applied urea–N in a soil-plant system [10,11]. The collective use of DCD and urea has proven useful because they simultaneously reduced N2O emission by 53% and CH4 emission by 22% and improved crop quality [12]. Li et al. observed that DCD and HQ reduced the N2O and CH4 emissions during rice growth [13]. Boeckx et al. found that the application of Urea (U) with HQ, U with DCD, U with HQ plus DCD decreased N2O emissions by 11, 47 and 62%, respectively, and CH4 emission by 30, 53 and 58% [14].
It is well established fact that the uses of nitrification inhibitors like DCD results in minimizing the nitrogen losses from soil thus improving crop yield by effective nitrogen utilization. Previous literature shows that no research is available on the effect of DCD on the nitrogen transformations under anaerobic conditions. Little information is available on the proper conditions and the concentrations for DCD application for effective results. Therefore, the present study aimed to investigate the effects of various DCD concentrations applied to different soil moisture conditions and how DCD affects soil nitrogen transformations.
Materials and Methods
Soil
Paddy soil of sandy texture (organic matter 21.3 g/kg, with initial pH 7.98, Total Nitrogen 1.27 g/kg, and Total Phosphate 0.73 g/kg) was collected from experiment farm of Zhejiang University, China. The soil samples were air-dried, ground and sieved through 2 mm mesh before experiment.
Treatments
A laboratory study was carried out to demonstrate the effects of various concentrations of DCD on nitrogen transformation and emission of N2O under various water conditions. There were 9 treatments (each in triplicates) including combinations of 3 different
Water-Filled Pore Space (WFPS or degree of saturation) conditions with 2 different DCD concentrations i.e. (1) 60 % WFPS (2) 60% WFPS + 0.5% DCD (3) 60% WFPS + 2% DCD (4) 95 % WFPS (5) 95 % WFPS + 0.5 % DCD (6) 95 % WFPS + 2 % DCD (7) 110 % WFPS (8) 110 % WFPS + 0.5 % DCD and (9) 110 % WFPS + 2 % DCD. Urea was applied at the rate of 16.5 mg per treatment which was equivalent to 154 mg N/kg soil. Low concentration of DCD was 0.0825 mg+0.5% of urea while high concentration of DCD was 0.33 mg + 2% of urea.
To each jar (600 ml capacity), 50 g dry soil was placed and adjusted its water content, sealed with a lid and was kept steady through weighing. The jars were incubated at 30 °C then DCD was added into the corresponding jars in a solution mixed with urea after 3-day of prior incubation.
The N2O gas monitoring was carried out at the end of prior incubation (before fertilization) and on days 1, 9, 11, 13, 16,19,22,26 days after fertilization. In addition, soil samples were collected on the days 1, 2,4,7,10,14 after fertilization to measure nitrogen forms, and the sample of the 4th day was collected to monitor the microbial growth.
Analytical procedures
Soil nitrogen: Soil was extracted with 2M KCl solution for 60 min (soil extract ratio=1:4). NH4 +, NO2 - and NO3 - were analyzed in the soil extract using UV 1206 spectrophotometer. NH4 + was determined by indigotine colorimetry, NO2 - was determined by Griess-Ilosvay colorimetry and NO3 - was determined by phenolic-2-sulfonic acid colorimetry.
N2O: N2O was measured with a gas chromatograph (Shimadzu GC-14A) equipped with a 63Ni Electron Capture Detector (ECD) and 1.0 m Porapac N columns. The column and detector temperatures were 85 °C and 350 °C, respectively, and the carrier gases were 5% methane and 95% argon (30 ml min-1).
All the methods used for the determination of nitrogenous species were standard methods [15].
Microbial growth: Most Probable Number (MPN) enumeration technique [16] was used to monitor the populations of nitrogen cycle bacteria to describe the microbial growth situation.
Results
Effects of DCD on the N2O emission
When lower concentration of DCD (0.5% of urea) in 95% WFPS was applied, the appearance of N2O peak was deferred for 3 days compared with the control (urea without DCD) (Figure 1), while the addition of high DCD concentration (2% of urea) resulted in the complete inhibition of N2O emission. A similar trend was noted for the other two WFPS treatments, which indicated that high concentration of DCD could strongly inhibit N2O emissions.
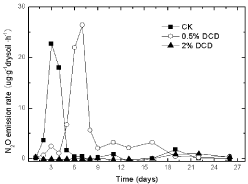
Figure 1: The effect of DCD on N2O emission rate with the passage of time
under 95% WFPS with urea.
Before DCD application, there was a little emission of N2O; however, no emission of N2O was observed after DCD application in 110 % and 95 % WSPF treatments. It seemed that no nitrate was available due to DCD application in 110% WFPS that might had changed to N2O, so it might contributed to absence of any N2O emission. Certain N2O emission under 60 % WSPF conditions was also observed (Figure 2).
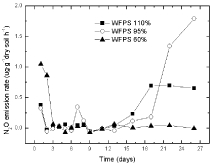
Figure 2: The variations of N2O emission in paddy soils with 2% DCD and
urea.
Effects of DCD on soil nitrogen transformations
Ammonium nitrogen: The DCD application could strongly affect the transformation of soil ammonium nitrogen. There was little discrepancy of ammonium nitrogen concentration between the lower and higher levels of DCD treatments under 60% and 95% WFPS conditions for the first 4 days of incubation (Figure 3 and 4). Thereafter, ammonium nitrogen concentration with 0.5% DCD application decreased much faster than that with 2% DCD application. Before the 4th day, the decrease of ammonium nitrogen was induced by ammonia volatilization due to the inhibition of nitrification. After the 4th day, the sharp decrease of ammonium nitrogen with 0.5% DCD application was mostly induced by nitrification caused by the complete decomposition of DCD. In the control treatment, little ammonium nitrogen accumulated because of efficient nitrification (Figure 5).
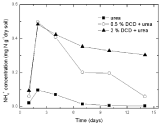
Figure 3: Change of NH4 + concentration for different DCD concentrations
under 110% WFPS.
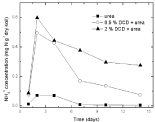
Figure : Change of NH4 +concentration in paddy soils with the passage of time.
The similar discrepancy between the lower and higher concentration DCD application appeared earlier under 60% WSPF condition which was probably because DCD might have easily decomposed by soil microbes under aerobic condition.
Nitrite nitrogenSoil nitrite concentrations increased from the 2nd day and reached at the peak value on the 7th day without DCD application under 95% and 110% WSPF conditions. With 0.5% DCD application, nitrite concentration increased from the 4th day and also reached the peak on the 7th day but with lower value (0.004-0.06 mgN/g dry soil) which was only 1/4 of that in the control (0.016- 0.029 mg N/g dry soil). The delayed increase of nitrite concentration at low applied DCD concentration seemed due to DCD mediated inhibition of the ammonium nitrogen transformation to nitrite at the early experiment stage that caused stunted increase of nitrite concentration. But with the disappearance of nitrification inhibition, nitrite concentration also increased. It was indicated that 0.5% DCD application could defer the appearance of peak value of N2O emission because it could inhibit soil nitrification for a short time.
At higher DCD applied level (Figure 6 and 7), nitrite concentrations kept very low throughout indicating that soil nitrification was completely inhibited thus nitrite concentration didn’t increase.
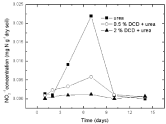
Figure 6: Change of NO2-1 concentration in paddy soils with different concentration DCD under 110% WFPS.
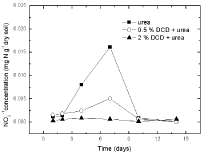
Figure 7: The change of NO2 -1 concentration in paddy soils with different
concentration DCD under 95% WFPS.
Under 60% WSPF condition (Figure 8), there was a similar trend in all treatments i.e. high concentration of soil nitrite before fertilization and persistent decrease after incubation. DCD could only inhibit the transformation from ammonium nitrogen to nitrite rather than that from nitrite to nitrate. Compared with the change of nitrite concentration with DCD application, it not only decreased but also increased during the first 2-4 days without DCD application. It was perhaps because soil nitrite mostly came from intrinsic soil nitrite pool or nitrification with DCD application and was supplemented by nitrification without DCD application.
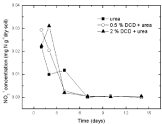
Figure 8: Change of NO2-1 concentration in paddy soils with different concentration DCD under 60% WFPS.
Nitrate nitrogen: Under 95% and 110% WSPF conditions, there was a little initial accumulation of soil nitrate and nitrate concentration increased from the 2nd day and the 4th day without DCD and with 0.5% DCD application, respectively, and both reached the peak value on the 10th day, but the peak value of nitrate concentration in 0.5% DCD treatment was lower over the control (Figure 9 and 10). With higher level of DCD application, nitrate concentrations kept very low all the time which indicated that soil nitrification was completely inhibited.

Figure 9: Change of NO3-1 concentration in paddy soils with different concentration DCD under 110% WFPS.
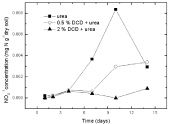
Figure 10: Change of NO3-1concentration in paddy soils with different concentration DCD under 95% WFPS.
Under 60% WSPF condition, a little soil nitrate accumulated before fertilization and continuously increased after only urea application and reached the peak on the 10th day, indicating that ammonium nitrogen in soil was continuously oxidized to nitrate due to nitrification (Figure 11). Although nitrate concentration increased in both applied DCD treatments, the increase was much slower over the control which was because the increase of nitrate concentration mostly induced only by nitrite oxidation.
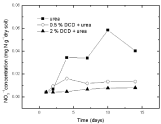
Figure 11: Change of NO3-1 concentration with different concentration DCD under 60% WFPS.
Effects of DCD on soil nitrifying and denitrifying bacteria
Nitrifying bacteria: The inhibition of low DCD concentration on soil Nitrosobacteria was strong enough to decrease about these microbial populations up to one magnitude; while high DCD concentration was much stronger enough to decrease Nitrosobacteria populations’ up to two magnitudes.
A little inhibitory effect of DCD was noted on soil nitrobacteria at low DCD concentration as nitrobacterial population counts did not show much change at 0.5% DCD over the control. High DCD concentration caused a decrease in nitrobacteria MPN (Figure 12).
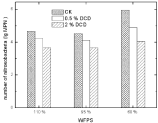
Figure 12: Effect of DCD to nitrosobacteria.
Denitrifying bacteria: There was no obvious effect of DCD on soil denitrifying bacterial MPN. The application of 0.5% DCD had nearly no effect on denitrifying bacterial counts.
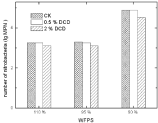
Figure 13: Effect of DCD to nitrobacteria.
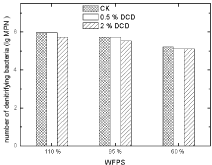
Figure 14: Effect of DCD on denitrifying bacterial numbers.
Discussion
Nitrification Inhibitors (NI) are chemicals designed to slow the process, reducing the risk that N will be lost through leaching and denitrification. It is well established that nitrification inhibitors like DCD can retain ammonium available to plant uptake; however, the characteristics of biophysical disappearance of DCD (i.e., biological decomposition, plant uptake and physical loss through surface runoff and leaching) and its longevity in soil are not well understood [17]. Kim et al. showed that 4~40% of applied DCD stayed on plant canopy for a duration of less than 6 days and up to 16 days and was affected by plant height and the timing of rainfall after DCD application [17]. Half life of DCD in soil was not affected by either the amount of DCD application (10 or 20 kg ha-1) or the source of N applied (synthetic fertilizer or urine) in a poorly drained New Zealand dairy grazed pasture soil. The present study showed that the application of lower concentration of DCD delayed the N2O emissions while higher concentration completely inhibited it. DCD, the dimeric form of cynamide with relatively high water solubility (23 g L-1 at 13°C), is receiving renewed interest, as it can move with fertilizers in the soil and can be dissolved in liquid manures [18]. It also contains about 65% N, is non-volatile, degrades to CO2, NH3 and H2O, and thus acts as a slow release N fertilizer. It is a bacteriostatic, non-toxic, chemical with LD50 of 10 g kg-1 body weight, which is about 3 times higher than NaCl [18]. The ammonium mono-oxygenase enzyme is suppressed by nitrification inhibitors, the enzyme that converts ammonium present of soil to hydroxyl amine which is oxidized to nitrite and then to nitrate [19]. The decreased availability of nitrite on the application of nitrification inhibitor might have reduced the nitrous oxide emissions in the present study.
Although low DCD concentration postponed the N2O emission, the peak value of its emission was not lower but even higher than the control value. Probably DCD could also be used by soil microbes as nitrogen source apart from its inhibition to soil nitrification [20], thus most of DCD was decomposed by soil microbial communities and part of it was transformed to N2O which led to the higher peak value of N2O emission than that of the control. As low DCD concentration could be decomposed by soil microbes in 3 days and consequently resulted in disappearance of N2O emission inhibition. It seemed that the accumulation of nitrite in soil before DCD application had induced the emission of N2O which indicated that DCD could only inhibit the prophase of soil nitrification, i.e. the step from ammonium nitrogen to nitrite rather than from nitrite to N2O. As DCD was probably decomposed by soil microbes at the end of the experiment, part of its inhibition on nitrification was relieved, peak values of N2O emission emerged at 95% and 110% WSPF conditions with high level DCD application. The decrease of ammonium nitrogen with 2% DCD application might be induced by ammonia volatilization due to the long-time inhibition of nitrification [21]. In the control treatment, little ammonium nitrogen accumulated because of efficient nitrification (Figure 5).
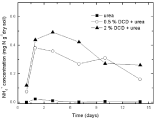
Figure 5: Temporal changes ofNH4 +concentration in paddy soils with different concentration DCD under 60% WFPS.
The DCD application could strongly affect the transformation of soil ammonium nitrogen. There was little discrepancy of ammonium nitrogen concentration between the lower and higher levels of DCD treatments under 60% and 95% WSPF conditions for the first 4 days of incubation (Figure 3 and 4). Thereafter, ammonium nitrogen concentration with 0.5% DCD application decreased much faster than that with 2% DCD application. Before the 4th day, the decrease of ammonium nitrogen was induced by ammonia volatilization due to the inhibition of nitrification. After the 4th day, the sharp decrease of ammonium nitrogen with 0.5% DCD application was mostly induced by nitrification caused by the complete decomposition of DCD.
The similar discrepancy between the lower and higher concentration DCD application appeared earlier under 60% WSPF condition which was probably because DCD was more easily decomposed by soil microbe under aerobic condition. DCD could only inhibit the transformation from ammonium nitrogen to nitrite rather than that from nitrite to nitrate. Compared with the change of nitrite concentration with DCD application, it not only decreased but also increased during the first 2-4 days without DCD application. It was perhaps because soil nitrite mostly came from intrinsic soil nitrite pool or nitrification with DCD application and was supplemented by nitrification without DCD application.
The inhibition of low DCD concentration on soil Nitrosobacteria was strong enough to decrease about these microbial populations up to one magnitude; while high DCD concentration was much stronger enough to decrease Nitrosobacteria populations’ up to two magnitudes. As far as the mechanism of DCD inhibition is concerned, it seems that cyanide group (–CN) of DCD reacted with sulfhydryl group (–SH) or metallic groups so that the structure or active site of the respiratory enzymes was so badly destroyed that their activities were weakened or even completely inhibited. Therefore, nitrosobacteria couldn’t gain energy for survival leading to sharp decrease of soil nitrosobacterial populations. The strong inhibitory effect of DCD on nitrosobacteria indicated that the first step of nitrification from ammonium nitrogen to nitrite was inhibited.
In a dry soil, nitrification may play a significant role in producing N2O from accumulated NH4 + during drainage period and denitrification may also generate N2O in deeper reduced soil layers and simultaneously in upper anaerobic micro sites [22]. In soil, nitrate denitrification could also be responsible for some N2O formation whenever the soil was made anaerobic by irrigating [23,24]. The variables including, type of inhibitors, the quantity applied and the method of inhibitor application, type of soil and water management practices of rice field, affect the inhibition efficiency of HQ and DCD. DCD was reported to reduce N2O emission by 40% in a dry in sandy loam, while no inhibition was noticed under wet conditions [25]. Pathak and Nedwell also indicated that DCD along urea significantly reduced the N2O emission to 63% over urea alone at the field capacity, but no inhibitory effect was evident under submerged conditions [26]. Majumdar et al. studied the effect of DCD at various application rates (5–25% of applied urea–N) on nitrification and N2O emission, indicating that DCD incorporated at the rate of 5 and 25% of applied urea–N were most effective in mitigating total N2O emission [2]. Watkins et al. did not notice any significant effect of DCD on denitrification or its enzymes [27].
The present study showed that at higher DCD applied level under fully moist conditions (110 % WSPF), nitrite concentrations kept very low throughout indicating that initial step of nitrification was completely inhibited thus nitrite concentration didn’t increase. It may thus be suggested that 2 % DCD may be applied under fully moist conditions to increase in rice yield and a reduction of total Global Warming Potential (GWP) of N2O could be achieved by using inhibitor(s) in rice paddy field.
Conclusion
Low level DCD application could defer the appearance of N2O emission peak for 3 days while high level DCD could strongly inhibit the emission of N2O. Under 95% and 110% WSPF conditions, soil nitrite concentration increased and reached the peak on the 7th day with 0.5% DCD application same as in the control, while it was very low all the time in the 2% DCD treatment. Strong inhibitory effects of DCD were noted on nitrosobacterial populations without any obvious influence on soil nitrobacteria and denitrifying bacteria. At higher DCD applied level under fully moist conditions (110 % WSPF), nitrite concentrations kept very low throughout indicating that initial step of nitrification was completely inhibited thus nitrite concentration didn’t increase. It may thus be suggested that 2 % DCD may be applied under fully moist conditions to increase in rice yield and a reduction of total Global Warming Potential (GWP) of N2O.
Acknowledgement
This study was financially supported by national natural science foundation of China (49901011) and provincial natural science foundation of Zhejiang (Y504247), major projects on control and management technology of water pollution of China (Grant No. 2009ZX07317-008).
References
- Majumdar D, Kumar S, Pathak H, Jain MC, Kummar U. Reducing nitrous oxide emission from an irrigated rice field of North India with nitrification inhibitors. Agriculture, Ecosystems and Environment. 2000; 81: 163–169.
- Majumdar D, Pathak H, Kumar S, Jain MC. Nitrous oxide emission from a sandy loam Inceptisol under irrigated wheat in India as influenced by different nitrification inhibitors. Agriculture, Ecosystems and Environment. 2002; 91: 283–293.
- Xing GX, Zhao X, Xiong ZQ, Yan XY, Xu H, Xie YX, et al. Nitrous oxide emission from paddy fields in China. Acta Ecologica Sinica. 2009; 29: 45-50.
- IPCC. Changes in atmospheric constituents and in radioactive forcing. Climate change. 2007.
- Wrage N,Velthof GL, van Beusichem ML, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biology and Biochemistry. 2001; 33: 1723-1732.
- Isermann K. Agriculture’s share in the emission of trace gases affecting the climate and some cause-oriented proposals for sufficiently reducing this share. Environmental Pollution. 1994; 83: 95–111.
- Cui M, Sun XC, Hu CX. Effective mitigation of nitrate leaching and nitrous oxide emissions in intensive vegetable production systems using a nitrification inhibitor, dicyandiamide. J Soils Sediments. 2011; 11: 722-730.
- Xu XK, Wang YS, Zheng XH, Wang MX, Wang Z, Zhou LK, et al. Methane emission from a simulated rice field ecosystem as influenced by hydroquinone and dicyandiamide. Science of the Total Environment. 2000; 263: 243–253.
- Xu XK, Boeckx P, Van Cleemput O, Zhou LK. Urease and nitrification inhibitors to reduce emissions of CH4 and N2O in rice production. Nutrient Cycling in Agroecosystems. 2002; 64: 203–211.
- Xu XK, Zhou LK, Van Cleemput O, Wang Z. Fate of urea-15N in a soil-wheat system as influenced by urease inhibitor hydroquinone and nitrification inhibitor dicyandiamide. Plant and Soil. 2000; 220: 261–270.
- Xu XK, Huang Y, Zhou LK, Huang GH, Van Cleemput O. Effect of dicyandiamide and hydroquinone on the transformation of urea-nitrogen-15 in soil cropped to wheat. Biol Ferti Soils. 2001; 34: 286–290.
- Ghosh S, Majumdar D, Jain MC. Methane and nitrous oxide emissions from irrigated rice of North India. Chemosphere. 2003; 51: 181–195.
- Li XG, Zhang XY, Xu H, Cai ZC, Yagi K. Methane and nitrous oxide emissions from rice paddy soil as influenced by timing of application of hydroquinone and dicyandiamide. Nutrient Cycling in Agroecosystems. 2009; 85: 31-40.
- Boeckx P, Xu XK, Van Cleemput O. Mitigating of N2O and CH4 emission from rice and wheat cropping systems using dicyandiamide and hydroquinone. Nutrient Cycling in Agroecosystems. 2005; 72: 41–49.
- Andrew DE, Lenore SC, Eugene WR, Arnold EG. Standard methods for the examination of water and wastewater. 21st edn. American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). 2005; 4-103—4-134.
- Rowe R, Todd R, Waide J. Microtechnique for most-probable-number analysis. Applied and Environmental Microbiology. 1977; 33: 675–680.
- Kim DG, Giltrap D, Saggar S, Palmada T, Berben P, Drysdale D. Factors controlling disappearance of nitrification inhibitor, dicyandiamide (DCD) in a grazed pasture soil in Manawatu. Currie LD, Christensen CL, editors. Advanced Nutrient Management: Gains from the Past-Goals for the Future, Massey University, Palmerston North, New Zealand. 2012.
- Amberger A. Research on dicyandiamide as a nitrification inhibitor and future outlook. Communication Soil Science and Plant Analysis. 1989; 20: 1933–1955.
- Prasad R, Power PJ. Nitrification inhibitors for the agriculture health and environment. Advances in Agronomy. 1995; 54: 233–281.
- Slangen JHG, Keerkhoff P. Nitrification inhibitors in agriculture and horticulture: a literature review. Fertilizer Research. 1984; 5:1–76.
- Rajbanshi SS, Benckier G, Ottow JCG. Effects of concentration, incubation temperature, and repeated applications on degradation kinetics of dicyandiamide (DCD) in model experiments with a silt loam soil. Biology and fertility of Soils. 1992; 13: 61–64.
- Arah JRM, Smith KA. Steady state denitrification in aggregated soils: a mathematical model. Journal of Soil Science. 1989; 40: 139–149.
- Zaman M, Nguyen ML, Blennerhassett JD, Quin BF. Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biology and Fertility of Soils. 2008; 44: 693-705.
- Boonlue K, Davey LJ, Nantana G, Gareth EJ, Atsamon L. Seasonal nitrous oxide emissions from different land uses and their controlling factors in a tropical riparian ecosystem. Agriculture, Ecosystems and Environment, 2012; 158: 15-30.
- Skiba U, Smith KA, Fowler D. Nitrification and denitrification as sources of nitric oxide and nitrous oxide in sandy loam soil. Soil Biology and Biochemistry. 1993; 25: 1527–1536.
- Pathak H, Nedwell DB. Nitrous oxide emission from soil with different fertilizers, water levels and nitrification inhibitors. Water Air and Soil Pollution. 2001; 129: 217–228.
- Watkins NL, Schippera LA, Sparlinga GP, Thorroldb B, Balksa M. Multiple small monthly doses of dicyandiamide (DCD) did not reduce denitrification in Waikato dairy pasture. New Zealand Journal of Agriculture. 2013; 56: 37-48.