
Research Article
Austin J Biotechnol Bioeng. 2016; 3(1): 1058.
Establishment of High Frequency Callus induction and Genetic Transformation in Neglected Leafy Vegetable Amaranthus trisis
Murugan SB1,2 and Sathishkumar R1*
1Department of Biotechnology, Bharathiar University, India
2Department of Biotechnology, P.S.R. Engineering College, India
*Corresponding author: Ramalingam Sathishkumar, Plant Genetic Engineering Laboratory, Department of Biotechnology, Bharathiar University, Coimbatore, Tamil Nadu, India
Received: February 17, 2015; Accepted: March 04, 2016; Published: March 07, 2016
Abstract
In the present study, an efficient in vitro callus induction and genetic transformation protocol was optimized for neglected leafy vegetable Amaranthus tristis. In vitro grown plant leaves were used as explants for callus initiation. Six different hormone combinations were tested for callus induction. Of the different hormone combination tested, Murashige and Skoog (MS) medium supplemented with 2, 4-dichlorophenoxyacetic acid and 6-benzylaminopurine (0.5mg/l) showed effective callus proliferation (87%). The frequency of callus induction ranged from 30.3% to 87%. Even though different hormone combinations were tried, the proliferated callus failed to differentiate into shoots. After 5th subculture, the callus was turned brown and dried. For genetic transformation, the plasmid pCAMBIA1301 harboring the GUS gene was transformed into the leaf explants by Agrobacterium mediated method. The presence of GUS gene in the putative transformed callus was confirmed by Polymerase Chain Reaction (PCR) and GUS histochemical staining. In PCR, an expected band of 1.2kb size was observed in all transformed calli confirming the presence of GUS gene. In histochemical staining, the transformed callus develops blue color in the presence of X- Gluc (5- Bromo-4- Chloro-3- Indolyl- Beta-D- Glucuronidase) reagent. Although shoot regeneration was unsuccessful, the present study might helps to produce secondary metabolites from callus suspension cultures in Amaranthus tristis. This study can also prelude for metabolic engineering studies in this neglected vegetable to meet our future nutritional requirements.
Keywords: Amaranthus trisis; Agrobacterium transformation; Callus; Histochemical Staining; PCR
Introduction
Leafy vegetables are known to possess several ethno-medicinal properties, hence they are regarded as “Nature’s Anti-aging Wonders” [1,2]. The leafy vegetable Amaranthus tristis belongs to the family Amaranthaceae. The plants of this family are herbaceous, hard and fast growing plants. The leaves of this plant are edible and known to possess numerous medicinal properties [3]. In Ayurvedic system of medicine, it is stated that Amaranthus has potential medicinal properties and various species of this plant are used for curing several diseases. A. tricolor is used for quick healing of wounds and A. spinosus is used for treating diarrhea [4,5]. A. tristis is used as an astringent in dysentery and also used for treating cough and bronchitis [3]. In order to meet the daily sustainable food requirements this vegetables should be included in our diet.
The genetic engineering of plants has become the core research in plant molecular biology. The transformation efficiency determines the success in transgenic research. The genetic transformation of plants with the aim to introduce specific traits in plants has led to the development of crops with high nutritional value, resistance to biotic and abiotic stresses [6,7]. Totipotency of plant cell makes the genetic transformation and regeneration of plants easy. For the large scale transformation, it is necessary for the stable integration of foreign gene in genome of the organisms. Different transformation protocols
have been used for different species [8]. There are several methods available for the genetic transformation in plants viz., Agrobacterium mediated, particle bombardment technology, microinjection, polyethylene glycol-mediated transfer and electroporation are the some of the methods used for the production of transgenic plants. Of these, Agrobacterium mediated and particle bombardment technology is the method of choice by most of the researchers for the genetic engineering experiments [9].
Establishment of in vitro system is necessary for both plant improvement and to conserve natural resources from extinction. As in vitro tissue culture was genotype dependent and also varies even between the cultivars, our aim was to optimize the protocol for high frequency callus induction and genetic transformation in Amaranthus tristis CO3, a valuable yet neglected leafy vegetable. As often vegetable crops are the ideal target for nutritional content improvement, this work could be a prelude for metabolic engineering approaches to meet our future nutritional requirements.
Materials and Methods
Surface sterilization and seed germination
The seeds of Amaranthus tristis variety CO3 were procured from Seeds Certification Centre, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu. The Amaranthus seeds were washed thrice thoroughly with sterile distilled water, surface sterilized using 0.1% mercuric chloride and further washed with sterile distilled water to remove traces of mercuric chloride. The surface sterilized seeds were blot dried on sterile filter paper and aseptically inoculated in MS medium devoid of any hormones. The seeds inoculated were kept in dark for 3 days and then incubated in the light for 16±8 hours.
Pre-culture of leaves and callus induction
In vitro grown plant leaves were used as explants in this study. The leaves were cut into small pieces and inoculated on pre-culture media. Six different hormone concentrations in different combinations were tried for effective callus initiation and proliferation. MS media supplemented with 2, 4-Dichlorophenoxyacetic Acid (2,4-D) and 6-Benzylaminopurine (BAP) (0.5 mg/L); Naphthaleneacetic Acid (NAA) and 6-Benzylaminopurine (BAP) (0.5 mg/L); 2,4-D and BAP (1mg/L), NAA and BAP (1mg/L), 2,4 –D and Kinetin (0.5 mg/L) and NAA and Kinetin (0.5 mg/L) were used. The leaves were pre-cultured on the above media for the callus induction.
Agrobacterium mediated genetic transformation using pCAMBIA 1301
The pre-cultured leaves were used as explants for the DNA delivery studies in order to optimize the genetic transformation in Amaranthus. Agrobacterium tumefaciens strain EHA 105 harboring pCAMBIA 1301 was inoculated in Luria Bertani (LB) medium containing appropriate antibiotics and incubated overnight at room temperature with shaking. After reaching the OD of 0.6 at 600nm, the cells were harvested and pelleted at 5,000 rpm for 10 minutes and resuspended in liquid MS medium. The explants were infected for 10-15 minutes; blot dried on sterile filter paper for 20 minutes and were transferred to co-cultivation medium and incubated in dark condition for 3 days. After the co-cultivation in dark for three days, the explants were blot dried on sterile filter paper and inoculated on selection medium. Selection media was the same as the propagation media supplemented with 100mg/L Hygromycin + 300mg/L Carbenicillin (Duchefa, Germany).
Screening of transformants
After two to three rounds of subculture in the selection medium, the genomic DNA from putative transformed callus was isolated [10]. In order to confirm the putative transformants, polymerase chain reaction was performed for GUS gene with gene specific primers. The GUS gene expression in transformed callus was analyzed by GUS histochemical staining. To the gently scrapped callus, X- Gluc (5- Bromo-4- Chloro-3- Indolyl- Beta-D- Glucuronidase) reagent was added and incubated in dark for 3-4 hours at 37oC. The transformed callus was observed for the development of blue color.
Results
Amaranthus seeds were surface sterilized and inoculated on MS medium. Inoculated seeds were germinated after 7 days. The in vitro grown leaves of A. tristis were used as explants for callus formation. MS medium supplemented with different hormones in different combinations were tested for effective callus initiation. The initiation of callus was observed on the cut ends of the leaves after 10 days of the culture. Callus induction frequency was highly varied between hormonal combinations tested. Of the different hormone concentration tested, MS medium supplemented with 2, 4-D and BAP (0.5mg/l) showed effective callus proliferation (87%) (Figure 1). The explants inoculated on MS medium with NAA and Kinetin (0.5mg/l) showed lowest callus induction percentage. The frequency of callus induction ranged from 30.3% to 87% (Table 1). Our results showed that the leaf explants of A. tristis cultured on MS medium supplemented either with 2, 4 D or NAA in combination with BAP produced more than 50% of callus. In all the tested combination, loose, white and rapid proliferation of callus was observed on callus induction medium after 3 weeks however no shoot differentiation and plant regeneration was observed. The necrosis was observed in the callus after 5th sub-culture and dried slowly with rooting.

Figure 1: Callus formation in MS media containing 0.5 mg/l 2,4-D and 0.5
mg/l BAP.
Explant used
Medium
Percentage of callus induction (%)
Amaranthus leaves
MS+2,4-D+BAP (0.5mg/l)
87±0.56
MS+NAA+BAP (0.5mg/l)
71.4±0.58
MS+2,4-D+BAP (1mg/l)
68.75±0.58
MS+NAA+BAP (1mg/l)
60.6±1.15
MS+2,4-D+Kinetin (0.5mg/l)
33.3±1.00
MS+NAA+Kinetin (0.5mg/l)
30.3±0.52
Table 1: Callus responses of Amaranthus tristis Variety CO3 in MS media with different hormonal combinations.
In order to optimize the DNA delivery efficiency in Amaranthus, Agrobacterium mediated transformation was performed. The pre-cultured Amaranthus leaves were used for Agrobacterium transformation. For Agrobacterium mediated transformation, the cocultivation period for 3 days has shown better results in Amaranthus. DNA isolated from putative transformed calli was confirmed by PCR by using GUS gene specific primers. The putative transformants were confirmed by the presence of specific band at 1.2kb which corresponds to GUS gene. The GUS gene expression was also confirmed by GUS histochemical staining (Figure 2 and 3).
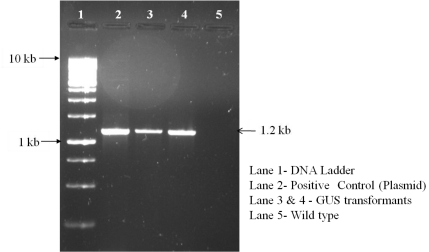
Figure 1: PCR Amplification of GUS Gene from the DNA isolated from
putative transformed callus.
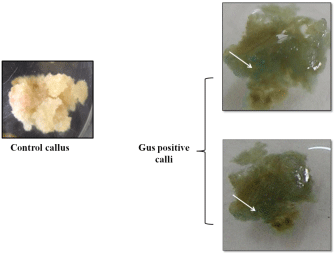
Figure 3: Histochemical Staining of GUS transformed callus. Arrow head
showing GUS expression in transformed callus.
Discussion
Amaranthus is one of the important leafy vegetable that has high nutritional value which can be found throughout the tropics [11]. As it is underutilized vegetable, development of in vitro culture might helpful for its improvement and genetic manipulation. The callus induction is considered as the most important initial step for plant genetic transformation. In our study, 2, 4 D or NAA in combination of BAP produce white, loose callus. 2, 4 D is considered to be effective in callus induction. The effective callus formation and regeneration is considered as species specific [12]. Jofre-Garfias et al. [13] reported that mature embryos of Amaranthus hypochondriacus cultured on MS medium supplemented with 10 μM 2, 4-dichlorophenoxyacetic acid or 3, 6-dichloro-2-methoxybenzoic acid and 10% coconut liquid endosperm showed effective callus proliferation and plant regeneration. In our study, after 5th culture, callus turned brown and dried. This might be due to the accumulation of phenolics which inhibits the plant cellular growth [14,15].
The pre-cultured leaves were used for genetic transformation. Our study proved that co-cultivation period of 3 days resulted in high transformation efficiency; however co-cultivation period of above 3 days resulted in overgrowth of Agrobacterium and co-cultivation period of below 3 days result in low transformation efficiency. It has been reported that co-cultivation time varies between different species. Cervera et al. [16] reported that the co-cultivation period of 5 days showed maximum transformation efficiency in citrange. Agrobacterium mediated transformation through floral dip method in Amaranthus was reported earlier [17]. The major advantage of Agrobacterium mediated transformation was the integration of target gene in the transcriptionally active regions of the chromosome and the integrated gene follows mendelian inheritance [18-20]. Initially this method was thought to be more suitable for dicotyledonous plants but this method has also been used to transfer the foreign gene in monocotyledonous plants like rice and banana [21,22]. Though there are advantages and disadvantages in all transformation methods, it is necessary to optimize the parameters based on the host system and the transformation method used. Once an effective transformation protocol can be optimized, the genetic manipulation can be made easy, more convenient and reproducible.
Conclusion
In conclusion, we have optimized the hormonal combination for efficient callus induction and genetic transformation for neglected leafy vegetable Amaranthus tristis which would be very useful for the genetic improvement studies. Further studies are essential to find out the optimum media and hormonal concentration for the efficient regeneration system which can be prelude for metabolic engineering studies in this neglected vegetable to meet our future nutritional requirements.
Acknowledgement
The authors are very thankful to, The Department of Biotechnology, Bharathiar University for supporting this research through UGC-SAP and DST-FIST funds.
References
- Gupta S, Prakash J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum Nutr. 2009; 64: 39-45.
- Murugan SB, Deepika R, Reshma A, Ashwini M, Sathishkumar R. Assessment of free radical scavenging activities of leaves and stem fractions of green leafy vegetables. Afr J Pharm Pharmacol. 2014; 8: 1138-1145.
- Murugan SB, Deepika R, Reshma A, Balamurugan S, Sathishkumar R. Antioxidant capacities of Amaranthus tristis and Alternanthera sessilis: A comparative study. J Med Plants Res. 2013; 7: 2230-2235.
- Buragohain J. Ethnomedicinal Plants Used by the ethnic Communities of Tinsukia District of Assam, India. Recent Res Sci Technol. 2011; 3: 31-42.
- Srivastava A, Patel SP, Mishra RK, Vasistham RK, Singh A, Piskar AK. Ethanomedicinal importance of plants of Amarkantak region, Madhya Pradesh, India. Int. J Med Arom Plants. 2012; 2: 53-59.
- Harish MC, Dachinamoorthy P, Balamurugan S, Murugan SB, Sathishkumar R. Enhancement of a-tocopherol content through transgenic and cell suspension culture systems in tobacco. Acta Physiol Plant. 2013; 35: 1121-1130.
- Shanmugaraj BM, Ramalingam S. Plant expression platform for the production of recombinant pharmaceutical proteins. Austin J Biotechnol Bioeng. 2014; 1: 4.
- Oktem HA, Mahmoudian M, Eyidogan F, Yucel M. Gus gene delivery and expression in lentil cotyledonary nodes using particle bombardment. Lens Newslett. 1999; 26: 3-6.
- Moeller L, Wang K. Engineering with precision; tools for the new generation of transgenic crops. Bio Science 2008; 58: 391-401.
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus 1990; 12: 13-15.
- Makinde EA, Ayeni LS, Ojeniyi SO. Morphological characteristics of Amaranthus cruentus L. as influenced by kola pod husk, organomineral and NPK fertilizers in Southwestern Nigeria. N Y Sci J. 2010; 3: 130-134.
- Saharan V, Yadav RC, Yadav NR, Chapagain BP. High frequency plant regeneration from desiccated calli of indica rice (Oryza sativa L.). Afr J Biotechnol. 2004; 3: 256-259.
- Jofre-Garfias AE, Villegas-Sepulveda N, Cabrera-Ponce JL, Adame-Alvarez RM, Herrera-Estrella L, Simpson J. Agrobacterium-mediated transformation of Amaranthus hypochondriacus: light- and tissue-specific expression of a pea chlorophyll a/b-binding protein promoter. Plant Cell Rep. 1997; 16: 847-852.
- Daayf F, El Bellaj M, El Hassni M, J’Aiti F, El Hadrami I. Elicitation of soluble Phenolics in date palm callus by Fusarium oxysporum f.sp. albedinis culture medium. Environ Exp Bot. 2003; 49: 41-47.
- Dubravina GA, Zaytseva SM, Zagoskina NV. Changes in formation and localization of Phenolic compounds in the tissues of European and Canadian Yew during dedifferentiation in vitro. Russ J Plant Physiol. 2005; 52: 672-678.
- Cervera M, Navarro L, Pena L. Agrobacterium–mediated transformation of citrange: factors affecting transformation and regeneration. Plant Cell Rep. 1998; 18: 271-278.
- Munusamy U, Abdullah S, Aziz M, Khazaai H. Female reproductive system of Amaranthus as the target for Agrobacterium-mediated transformation. Adv Biosci Biotechnol. 2013; 4: 188-192.
- Rhodora RA, Thomas KH. Agrobacterium tumefaciens-mediated transformation of Japonica and India rice varieties. Planta 1996; 199: 612-617.
- Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the "gene-jockeying" tool. Microbiol Mol Biol Rev. 2003; 67: 16-37, table of contents.
- Kim SI, Veena, Gelvin SB. Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J. 2007; 51: 779-791.
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994; 6: 271-282.
- Cheng M, Lowe BA, Spencer TM, Ye X, Armstrong CL. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell Dev-Pl. 2004; 40: 31-45.