
Research Article
Austin J Biotechnol Bioeng. 2017; 4(4): 1087.
Effects of Acrylamide on Cervical Cancer (HeLa) Cells Proliferation and Few Marker Enzymes
Mallepogu V1, Jayasekhar Babu P2, Doble M2, Suman B1, Nagalakshmamma V3, Chalapathi PV3 and Thyagaraju K,1*
¹Department of Biochemistry, SVU College of Pharmacy, Sri Venkateswara University, India
²Bio Engineering and Drug Design Laboratory, Department of Biotechnology, Indian Institute of Technology, India
³Department of Chemistry, S.V Arts College (TTD), Sri Venkateswara University, India
*Corresponding author: Thyagaraju K, SVU College of Pharmacy, Sri Venkateswara University, Tirupathi-517502, AP, India
Received: October 12, 2017; Accepted: December 21, 2017; Published: December 28, 2017
Abstract
Acrylamide is known to be a genotoxic and carcinogenic compound, produced during food processing and has neurotoxic effects in humans. The aim of the present study is to investigate its effects on HeLa cancer cell lines, in terms of cytotoxicity, cell proliferation and morphological changes. Furthermore, we also investigated the influence of acrylamide on the levels of intracellular Malondialdehyde (MDA), Glutathione (GSH) and Glutathione S-Transferase (GST) activity. Our present study suggested that the acrylamide significantly increased the levels of Malondialdehyde (MDA), also remarkably decreased the level of Glutathione (GSH) and the activity of glutathione S-transferase. The acrylamide has reduced the number of viable HeLa cells by reducing its proliferation and inducing Caspase-3 enzyme activity, and apoptotic cell death. Though HeLa cells are cancerous the functions of acrylamide is almost identical to normal cell. Hence, it is reasonable to conclude that the acrylamide inhibits the growth of HeLa cells by inducing apoptosis.
Keywords: Acrylamide; Apoptosis; Caspase-3 enzyme; Cytotoxicity; HeLa Cancer Cell Lines
Introduction
Acrylamide is known as a carcinogen in experimental animal studies, and is classified by the International Agency for Research on Cancer (IARC) as a probable human carcinogen [1]. Importantly, the concentrations of acrylamide in foods are higher (30-2300μg/kg) than those of other carcinogens [2]. A prospective epidemiological study has found that, increased dietary intake of acrylamide is associated with increased risks of postmenopausal endometrial and ovarian cancer, particularly among non smokers [3]. This was further supported by a large prospective cohort study among women in the U.S [4]. They observed that risk for endometrial cancer and possibly ovarian cancer was greater among high acrylamide consumers. In addition, a positive association between dietary acrylamide intake and renal cell cancer risk was observed in a prospective cohort study [5]. Acrylamide is known to be neurotoxic, genotoxic and carcinogenic and has capacity to induce tumors in multiple organs of animals [6,7]. Increasing incidences of endometrial, ovarian, and renal cancer (but not brain cancer) with increased dietary acrylamide intake have been reported in humans [8].
The acrylamide induced oxidative stress, and it can be characterized as an imbalance between the levels of antioxidants and oxidants within the cell caused by age, environmental stressors, or diseases which lead to a deficiency in endogenous antioxidants, the excess production of Reactive Oxygen (ROS) and/or Reactive Nitrogen Species (RNS), or the reduced clearance of damaged proteins within the cell. Oxidative stress occurs due to excess of Reactive Oxygen Species (ROS). It is produced due to hydroxyl radical, superoxide anion, and hydrogen peroxide. These Reactive Oxygen Species (ROS) can lead to the destruction of cellular macromolecules, including lipids, proteins, and DNA, and ultimately can lead to cell death via apoptosis [9,10]. In apoptosis there are, extrinsic and intrinsic major pathways involved in the activation of caspases [11]. The extrinsic pathway is triggered by Fas and Tumor Necrosis Factor (TNF) through death receptors, and leads to the activation of initiator caspase 8 followed by cleavage of downstream effector caspases. The intrinsic pathway is induces the releasing of cytochrome-c from mitochondria and results in the activation of the initiator caspase 9, which then cleaves pro-caspase 3 for caspase-3 activation [12]. In the present experiment, we examined the cytotoxicity of acrylamide, identification of antioxidant defence and apoptotic cell death in HeLa cells.
Materials and Methods
Materials
The chemicals purchased from Merck, India and Hi Media, India and used for the analysis of various samples of our research. HeLa (Human cervical cancer cell line) cells were obtained from National Centre for Cell Sciences (NCCS), Pune, India.
Cytotoxicity assay
The Minimal Essential Medium (MEM), containing 1.0 mmol/L C3H3NaO3, 0.1 mmol/L nonessential amino acids, 1.5 g/L NaHCO3, 2 mmol/L L-glutamine supplemented with 10% fetal bovine serum (FBS; heat inactivated) and 1% antibiotic-antimycotic solution (1000 U/mL penicillin G, 10 mg/mL streptomycin sulphate, 5 mg/mL gentamycin, and 25 μg/mL amphotericin B), was used to maintain the HeLa (human cervical cancer) cells. The cells were cultured at 37°C in a humidified incubator (Heal Force, HF 160W, China) supplemented with 5% CO2. The cytotoxicity of acrylamide on cancer cells was evaluated by the MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay, which is a widely used screening method to measure cell viability and proliferation. The monocultures of the HeLa cells were incubated with increasing concentrations of filter (0.2 μm) sterilized acrylamide for 24 h. The cell viability was estimated by MTT dye conversion assay. Cells not exposed to acrylamide were considered as control. About 1x104 cells were seeded and maintained in a 96-well plate (Cell Bind, Corning) using MEM containing serum. After 24 h of incubation, the medium was replaced with serum free medium containing various concentrations of acrylamide (1- 6 mg/ml). The media was removed after 24 h of treatment and cells were washed with phosphate-buffered saline (PBS; 0.01 mol/L, pH = 7.2) followed by the addition of 100 μL of MTT (0.5 mg/mL) prepared in serum free medium to each well and incubated for 4 h in an incubator. Subsequently, medium was removed and 100 μL of Dimethyl Sulphoxide (DMSO) was added to each well to solubilize the formazan crystals. The concentration of formazan was determined using a multiwell plate reader (Tecon micro-plate reader, model 680, CA, USA) at 570 nm absorbance.
The cell viability was calculated with the following equation:
Cell viability (%) = Atreated /Acontrol X 100
Where A is the absorbance of cells at 570 nm.
Lipid peroxidation assay
HeLa cells were seeded in 10-centimetre Petri dishes. After 24-h of incubation, they were treated with acrylamide. As in control, cells were treated either with 8 or 80 mM hydrogen peroxide. Thereafter, the cells were scraped, washed twice with PBS, lysed in 520 mL of potassium chloride (1.15 %) for 30 min, and centrifuged at 5000 rpm. A volume of 500 mL of the supernatant was incubated with 2 mL of TBA (100 g/L) for 15 min at 100°C, cooled to room temperature, and centrifuged at 1000 rpm for 10 min. The volume of 2.5 mL of the supernatant, obtained in the previously described manner, was mixed with 1 mL of 0.8 % Trichloroacetic Acid (TCA) and incubated for 15 min at 100°C. After cooling with tap water, the absorbance of the samples was determined both at 532 and 600 nm (the latter concerns non-specific absorbance). The concentration of MDA-TBA complex, as an indicator of lipid peroxidation, was calculated from standard curves [13,14]. The concentration of cellular proteins was determined according to the Bradford method [15]. The results are expressed as concentrations of TBA-MDA complex (nano moles per mg of protein). Each experiment was repeated three times.
Determination of glutathione level
Intracellular Glutathione (GSH) content was examined spectrophotometrically, according to the procedure developed by Tietze, 1969 [16]. Cells were seeded in 10-centimetre Petri dishes. After 24h incubation, cells were incubated with 1-6mg/ml concentrations of the acrylamide and incubated for the next 24-h. Thereafter, cells were collected, counted, lysed and centrifuged at 12,000 rpm for 15 min. The GSH was determined in supernatants following the reaction with 5,5’-dithio-bis-(2-nitrobenzoic acid). The formation of 2-nitro- 5-thiobenzoic acid, which absorbs at 412 nm, was monitored. The results are expressed as GSH concentrations (μM/mg protein). The measurements of GSH concentrations were performed by Durgo et al. 2009 [17] in triplicates for each treated Petri dish.
Determination of glutathione S-transferase activity
Glutathione-S-transferase activity was assayed by the conventional method of Habig et al, 1974 [18]. One unit of enzyme activity was expressed as micromoles of GSH conjugate formed per milligram of protein and the increase in absorbance was read at 340 nm using 1-cholro 2,4 dinitro benzene as substrate.
Caspase-3 activity assay
Caspase-3 is a key biomarker for apoptosis. In vitro caspase-3 activity was measured using a colorimetric assay kit (Genscript, NJ, USA). As per the manufacturer’s instruction, HeLa cells incubated in DMEM medium containing 0.2% FBS were treated with acrylamide with 1-6mg concentrations for 24-h. The cells were then lysed to allow for detection of chromophore P-Nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA. The absorbance was measured at wavelength of 405 nm with a microtiter plate reader. The relative increase in caspase-3 activity was determined by comparing the absorbance of pNA from the acrylamide-treated HeLa cells with that from the nontreated control.
Morphology of HeLa cells
HeLa cells were seeded in 96-well culture plates. The acrylamide was added to the wells at concentrations of 1, 2, 3, 4, 5 and 6 mg in DMEM medium containing 0.2% FBS for 24-h. The morphologic changes of the cells were then observed under an inverted optical microscope (CKX41 Olympus; Olympus, Japan).
Determination of apoptosis by Double staining with annexin V-FITC and propidium iodide
The induction of apoptosis by acrylamide was evaluated by double staining of annexin V-FITC and Propidium Iodide (PI). After HeLa cells were incubated with 1-6mg acrylamide in DMEM medium containing 0.2% FBS in 6-well plates for 24-h, the cells were harvested, washed twice with cold PBS, and assayed for apoptosis by the double staining of annexin V-FITC and PI (Annexin V-FITC Apoptosis Detection Kit; KeyGEN, Nanking, China). Briefly, 5 × 105 cells were resuspended in a binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 1 mM MgCl2, 5 mM KCl, 2.5 mM CaCl2), stained with 5 μl of annexin V-FITC for 10 min, and then stained with 5 μl of PI for another 15 min. The cells were then immediately analyzed with a flow cytometer (FACScan; BD Biosciences, Milano, Italy). 3.9. Statistical analysis
Results were expressed as the means ± Standard Deviation (SD). Differences between groups were evaluated by using one-way ANOVA, followed by Duncan’s test. All statistical analyses were performed using the statistical software SPSS 11.0 (SPSS Ltd., Surrey, UK).
Results
The cytotoxicity of acrylamide under in vitro conditions in HeLa cells was examined by MTT assay for 24-h in terms of its effect on cell proliferation. HeLa cells were treated with the acrylamide ranging from 1-6 mg/ml. Only cells that are viable after 24-h of exposure to the acrylamide can metabolize MTT efficiently and produce purple colored crystal which is soluble with the addition of DMSO. Figure 1 shows the significant death of HeLa cell lines after 24-h post treatment and showed poor viability at concentrations starting from 2 mg/ml of acrylamide. As the concentration of acrylamide was increased from 1 to 6 mg/ml, the viability of HeLa cells in 24-h decreased from 76% to 30% in a dose dependant manner.
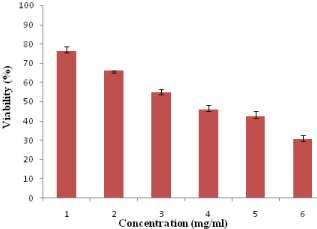
Figure 1: Cytotoxicity assay: viability of HeLa cells exposed to different
concentrations of acrylamide (1–6 mg/ml) over a period of 24-h.
Lipid peroxidation analysis
The toxic effect of the acrylamide was measured interms of the formation free radicals after treatment and was evaluated by lipid peroxidation assay, measured the amount of Malondialdehyde (MDA) formed in the HeLa cells.
Extreme and significant changes in MDA levels were observed in the acrylamide treated HeLa cells for 24-h when compared to control cells (13.3+0.1) (Figure 2). The MDA levels increases from 14.6±0.3 moles/mg protein to 32.4±0.4 moles/mg protein when the acrylamide concentration was increased from 1-6mg/ml in a dose dependent manner.
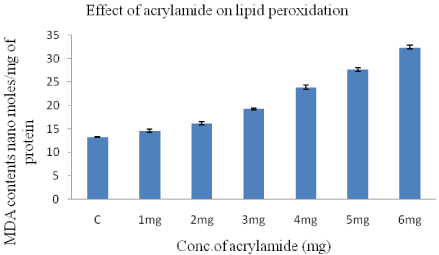
Figure 2: Concentration of TBA-MDA complex in HeLa cells after 24-h
treatment with acrylamide concentration varying from 1-6mg/ml.
Determination of total cellular glutathione Level
The effect of acrylamide on the levels of glutathione (reduced) hosted by HeLa cells are shown in (Figure 3). Our results showed that GSH levels reduced with increasing doses of acrylamide, in HeLa cells in a dose dependent manner when compared to control with 6 mg acrylamide in comparison with control (22.8±0.4). The GSH levels decreased from 21.4±0.2 to 6.1±0.1 when acrylamide concentration was increased from 1-6mg/ml.
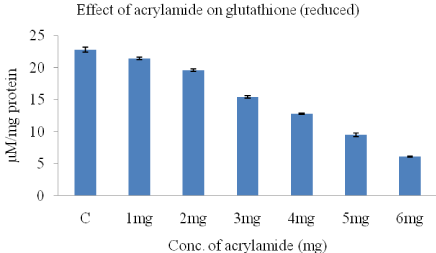
Figure 3: The concentration of glutathione in HeLa cells, obtained after 24-h
treatment with acrylamide.
GST activity analysis
The GST activity in HeLa cells reduced with increasing doses of acrylamide. The lowest GST activity as seen with 6mg acrylamide when compared to control (0.89+0.02). There is a dose dependent decrease in GST activity with increasing acrylamide concentration (Figure 4).
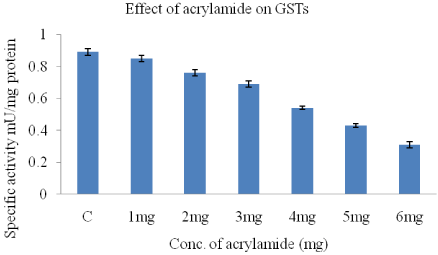
Figure 4: Effect of acrylamide on GST activity in HeLa cells.
Effect of acrylamide on caspase-3 enzyme activity in HeLa cells
The enzyme Caspase-3 in HeLa cells is activated by acrylamide in a dose-dependent manner (Figure 5). In every the concentration acrylamide from 1-6mg increases caspase-3 activity from 0.204±0.02 to 0.562±0.03. The caspase-3 activity in control is 0.198+0.02.
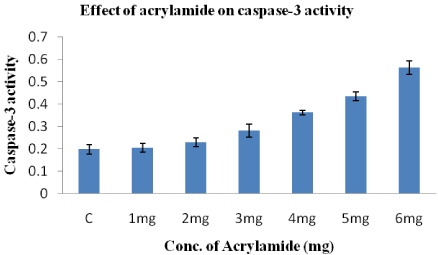
Figure 5: Effect of acrylamide on Caspase-3 enzyme activity after treatment
of 24-h. Acrylamide induces morphologic changes of HeLa cells.
Increasing the concentrations of acrylamide leads to a decrease in the numbers of the HeLa cells after 24-h (Figure 6). The changes in the cellular morphology after treatment with acrylamide suggest that the chemical induces cytotoxic effect causing significant damage or death to the cells. These results clearly demonstrate that acrylamide is toxic to the HeLa cells.
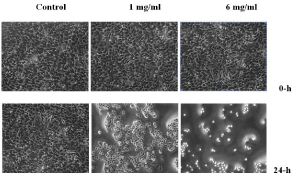
Figure 6: Morphology of the HeLa cells under an Inverted microscope.
Analysis of apoptosis by double staining HeLa cells with annexin V-FITC and PI
HeLa cells became apoptotic after they were treated with acrylamide. The distribution of apoptotic HeLa cells as measured by flow cytometre indicated that the numbers of early and late once cells significantly increased in comparison to the control group (not shown). As the concentration acrylamide is increased from 1-6mg, the percentage of apoptotic to total HeLa cells increased from 14 to 72.4±2.8 respectively (Figure 7).
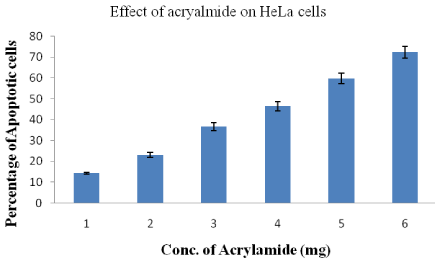
Figure 7: Percentage of apoptotic HeLa cells after treatment with different
concentrations of acrylamide.
Discussion
HeLa cancer cell lines are used for conducting a multitude of medical experiments, if the cells died, they could simply be thrown away and the experiment could be repeated on new cells without the potential loss of human life. This represented an enormous boon to medical and biological research [19]. These cells proliferate abnormally and rapidly, when compared to other cancer cells. The Reactive Oxygen Species (ROS) production induced by acrylamide and leading to cellular damages has been reported in several in vitro and in vivo studies [20].
The toxicity mechanism of all of these oxidants involves oxidative stress and impairment in the antioxidant defence system [21]. The genotoxicity and cytotoxicity of acrylamide causes disturbances in the antioxidant enzymes and non enzymes including GSH and ascorbic acid [22] which in turn damages the oxidative defence system in cells, leading to the release of ROS [23,24]. Oxidative stress has been proven to be involved in mutation, chromosomal aberration, tumour promotion, and cancer development. Oxidative stress has also been considered as an important mechanism of indirect genotoxicity. Antioxidant enzymes such as SOD, GSTs, CAT and GPx can protect cellular compounds against damage induced by free radicals. Therefore, the activities of these enzymes have been used to assess the oxidative stress in cells [25,26]. Over the past decade, the concept of programmed cell death has been broadened to more than ten types of cell death mechanisms that have been thoroughly characterized from both a morphological and a molecular point of view [27]. Apoptosis, thus far the best characterized type of programmed cell death, is believed to be the major obstacle to cancer growth through the elimination of damaged cells. For this reason, pro-apoptotic drugs have been extensively employed in anticancer therapies.
The present study used HeLa cells as an in vitro model, and the results revealed that the caspase-3 cleavage and the role of acrylamide is evident during apoptosis. Acrylamide has been reported to induce apoptosis in various types of cell models and laboratory animal organs [28]. Subchronic exposure to acrylamide affects the expressions of early molecular regulatory death-related proteins in the nervous systems of rats. The present study demonstrated that the acrylamide is able to induce apoptosis in HeLa cells. We tested the apoptotic effects of acrylamide on HeLa cells with various assays and the morphological alterations of the cellular nuclei are observed under an inverted microscope (Figure 6). The Caspase-3 activity, known to mediate integral parts of the apoptotic pathway [29-31] and is also shown to increase during acrylamide treatment. The high doses of acryalmide toxicity induced the oxidative stress with the loss of free radical scavenging activity. The antioxidant enzymatic activities reduced with increasing doses of acrylamide as well as decreased activitiy of glutathione-S-transferase (Figure 4) and glutathione level (Figure 3) which indicates oxidative stress and inhibition of antioxidant defense. The results from above studies demonstrated the apoptotic effect of acrylamide on HeLa cells.
Conclusion
We investigated the toxic effect of the acrylamide on the HeLa cells. Acrylamide induces the apoptosis in the HeLa cells after the treatment and was determined by caspase activity assay. We observed the increased caspase activity in treated cells suggesting the cell under apoptosis mechanism. We also found a correlation between time of exposure to acrylamide and the decrease in cell viability and its proliferation. Our results demonstrate that acrylamide is able to inhibit the growth of human cervical cancer cell line in vitro mechanism of inducing apoptosis it may gives an basis extended research for further examinations. These results made us investigation.
Acknowledgment
This work was supported financially by University Grants Commission (UGC), New Delhi, India, under the Post Doctoral Fellow (UGC-PDF) scheme.
Declaration of Interest
The authors declare that there is no conflict of interest and all authors have read and approved the text and consent to its publication.
References
- IARC. Acrylamide IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer. 1994; 60: 12-14.
- SNFA. Acrylamide is formed during the preparation of food and occurs in many foodstuffs. Swedish National Food Administration, Uppsala (2002).
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, Van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiology, Biomarkers and Prevention. 2007; 16: 2304–2313.
- Wilson KM, Mucci LA, Rosner BA, Willett WC. A prospective study on dietary acrylamide intake and the risk for breast, endometrial, and ovarian cancers. Cancer Epidemiology, Biomarkers and Prevention. 2010; 19: 2503–2515
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, Van den Brandt PA. Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. American Journal of Clinical Nutrition. 2008; 87: 1428–1438.
- Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol. 1995; 27: 95–105.
- Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, et al. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol. 1986; 85: 154–168.
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, Van den Brandt PA. Dietaryacrylamide intake and brain cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009; 18: 1663–1666.
- Rodriguez H, Holmquist GP, D’Agostino R, Keller J, Akman SA. Metal iondependent hydrogen peroxide-induced DNA damage is more sequence specific than metal specific. Cancer Res. 1997; 57: 2394–2403.
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006; 97: 1634–1658.
- Strasser A, O’ Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000; 69: 217–245.
- Nagata S. Apoptotic DNA fragmentation. Exp Cell Res. 2000; 256: 12–18.
- Cereser CS, Boget P, Parvaz A. Revol, Thiram-induced cytotoxicity is accompanied by a rapid and drastic oxidation of reduced glutathione with consecutive lipid peroxidation and cell death. Toxicology. 2001; 163: 153– 162.
- McCarthy SM, Somayajulu M, Sikorska H, Borowy-Borowski S, Pandey. Paraquat induces oxidative stress and neuronal cell death; Neuroprotection by water soluble coenzyme Q-10, Tox. Appl Pharmacol. 2004; 201: 21–31.
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 72: 248–254.
- Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969; 27: 502–522.
- Ksenija D, Vukovi L, Rusak G, Osmak M, Franeki J. Cytotoxic and Apoptotic Effect of Structurally Similar Flavonoids on Parental and Drug-Resistant Cells of a Human Cervical Carcinoma. Food Technol. Biotechnol. 2009; 47: 356–363.
- Habig WH, Jacoby WB. Assays for determination of glutathione S-transferases. Methods Enzymol. 1981; 77: 398–405.
- Batts DW. “Cancer cells killed Henrietta Lacks – then made her immortal”. The Virginian-Pilot. 2010; 1: 12-14.
- Naruszewicz M, Zapolska-Downar D, Kosmider A, Nowicka G, Kozlowska- Wojciechowska M, Vikstrom AS. Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: a pilot study. Am J Clin Nutr. 2009; 89: 773– 777.
- Veena S, Arti S, Leena K. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol. 2010; 48: 928–936.
- Thyaga Raju K, Venkataswamy M, Subbaiah KV, Suman B, Meenabai M, Rao KJ. Depletion of vitamin-c and glutathione by acrylamide Causes damage to hippocampus region of brain in chick Embryo. International Journal of Advances in Pharmaceutical research. 2013; 4: 1471–1479.
- Zamorano-Ponce E, Morales C, Ramos D, Sepulveda C, Cares S, Rivera P, et al. Anti-genotoxic effect of Aloysia triphylla infusion against acrylamideinduced DNA damage as shown by the comet assay technique. Muta Resgen Tox En. 2006; 603: 145–150.
- Liping J, Jun C, Yu A, Chengyan G, Shuxian Q, Lijie J, et al. Genotoxicity of acrylamide in human hepatoma G2 (Hep G2) cells. Toxicol In Vitro. 2007; 21: 1486–1492.
- Liu CM, Ma JQ, Lou Y. Chronic administration of troxerutin protects mouse kidney against D-galactose-induced oxidative DNA damage. Food Chem Toxicol. 2010a; 48: 2809–2817.
- Liu CM, Ma JQ, Sun YZ. Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environment Toxicol Phar. 2010b; 30: 264–271.
- Kornienko A, Mathieu V, Rastogi SK, Lefranc F, Kiss R. Therapeutic Agents Triggering Nonapoptotic Cancer Cell Death. J Med Chem. 2013; 56: 4823-4839.
- Lee JG, Wang YS, Chou CC. Acrylamide-induced apoptosis in rat primary astrocytes and human astrocytoma cell lines. Toxicol In Vitro. 2014; 28: 562–570.
- Lu JS, Ip HF, Lo SW, Wu C, Lin CC, Tang JP, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010; 33: 1181–1191.
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3- dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001; 61: 1862–1868.
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011; 351: 41–58.