
Review Article
J Blood Disord. 2014;1(3): 5.
Mesenchymal Stromal Cells: From Bone Marrow to Neoplastic Disorders
Giuseppina Divisato and Fernando Gianfrancesco
Institute of Genetics and Biophysics "Adriano Buzzati-Traverso", National Research Council of Italy, Italy
*Corresponding author: Fernando Gianfrancesco, Institute of Genetics and Biophysics "Adriano Buzzati- Traverso", National Research Council of Italy, Naples, Italy.
Received: October 20, 2014; Accepted: November 06, 2014; Published: November 07, 2014
Abstract
Mesenchymal stromal cells (MSCs) represent a small and heterogeneous subpopulation of mesenchymal stem cells that possesses multilineage differentiation potential. These cells are mainly present in bone marrow, but also in other tissues, and represent a valuable resource for their ability to differentiate into different cell lines and for many therapeutic approaches. MSCs are able to differentiate into cells of mesodermal origin such as adipocytes, chondrocytes, osteoblasts or fibroblasts and in vitro also into cells of non-mesodermal lineages. In bone marrow, they establish the microenvironment for the growth and differentiation of the hematopoietic stem cells (HSCs) resulting crucial for HSC maintenance and haematopoiesis. Nevertheless, the proliferation and/or the survival rate of MSCs may contribute to the onset of different types of bone sarcomas, such as Osteosarcoma, Chondrosarcoma and Giant Cell Tumor of Bone that represent the result of neoplastic degeneration of their corresponding committed mesenchymal precursors, probably as a consequence of the alteration of different or common biochemical pathways.
Keywords: Mesenchymal stromal cells; Bone marrow; Neoplastic disorders; Stem cells; FGFRs
Bone Marrow and Stem Cell Niches
Bone plays an essential role in the structure and movement of the body, and consists of cells (osteoclasts and osteoblasts) at different developmental stages, collagen fibrils, and mineral deposits such as calcium and phosphate. The bone cavity is filled with soft bone marrow that is the primary postnatal site of several stem cells including those of haematopoietic (HSC) and mesenchymal (MSC) lineages [1-5]. The stem cell niche represents the microenvironment created by supporting cells and their signals, in which stem cells reside and undergo self-renewal and differentiation [6-7]. The ability of adult stem cells to self-renew and differentiate is a critical point for tissue homeostasis: the depletion of this population occurs as a consequence of boosted self-renewal rate. On the other hand, the uncontrolled expansion of stem cell population, could promote tumorigenesis. The quiescence of stem cells within the niche is essential, about 70% of which are in the G0 phase of the cell cycle. Particularly, it has been shown that approximately 30% of the quiescent HSC divide every 145-193 days (protecting them from DNA damage by limiting the number of their cellular divisions), while a more active subpopulation divides every 28-36 days [8]. One mechanism that ensures the balance between self-renewal and differentiation processes is the control of asymmetric/symmetric stem cell division. In asymmetric division the stem cells divide into 2 daughter cells: one daughter cell remains in the niche as a stem cell and the other leaves the niche to produce a committed cell population. In symmetric division stem cells divide into 2 identical daughter cells that remain both in the niche as stem cells. The last decade has witnessed an increasing interest in stem cell niches, even if the numbers of niches in bone marrow, their cellular composition as well as their interactions are yet to be clearly determined [6,9-15]. The biochemical pathways promoting MSCs commitment are activated by micro-environmental conditions that include hormones (PTH, vitamin D3 and estrogen), growth factors (BMPs, TGFβs, IGF), and mechanical stimuli. In addition, there is an increasing interest in the role of non-coding RNAs (miRNAs) as well as of epigenetic mechanisms regulating the differentiation fate of MSC [16-18].
Mesenchymal Stromal Cell Differentiation
Haematopoietic stem cells mainly reside within the bone marrow, which is the primary site of HSC maintenance and haematopoiesis and also contains many other different non-haematopoietic cell types (Figure 1). Stromal and differentiated cells (chondrocytes, osteoblasts, fibroblasts, adipocytes) compose this microenvironment, normally referred as stroma [19,20]. Particularly, mesenchymal stromal cells that are committed toward osteoblast lineage express bone sialoprotein, osteonectin, osteopontin, osterix and Runx2 (runt-related transcription factor 2) [21]. Many different biochemical pathways drive this osteoblast differentiation program including FGFs (fibroblast growth factors) and WNTs signaling [22,23]. Specifically, FGF receptor ligands are involved in proliferation and osteoblast differentiation of mesenchymal precursors and therefore in bone deposition, through the interaction with four type of fibroblast growth factor receptors (FGFRs) [24,25]. FGFR3 and FGFR4 are involved in the differentiation of chondrocytes. On the other hand FGFR1 is involved in osteoblast proliferation while FGFR2 promotes osteoblast differentiation of mesenchymal stem cells promoting Runx2 expression and inhibiting TWIST1 [26,27]. Also Wnt signalling is involved in MSCs proliferation and in the regulation of the osteogenic differentiation [28]. Canonical Wnt signaling can be activated by the interaction of several secreted Wnt ligands with frizzled receptors and with the co-receptor Lipoprotein Receptor Related Protein 5/6 (LRP5/6). Wnt activation, involves different effectors such as β-catenin, JNK and calcium-channels regulators. Cytoplasmatic β-catenin accumulation and its nuclear translocation promote the activation of several oncogenes (e.g., c-Myc) and of different metalloproteinases, promoting extracellular matrix (ECM) degradation and cellular invasion and migration [29]. Wnt3a ligand has a modulatory function in chondrogenesis through bone morphogenetic protein (BMP)-2 expressions; Wnt7a enhances chondrogenesis through various TGFB1-MAPK signaling pathways; and Wnt1 inhibits chondrogenesis promoting TWIST1 up regulation [30].
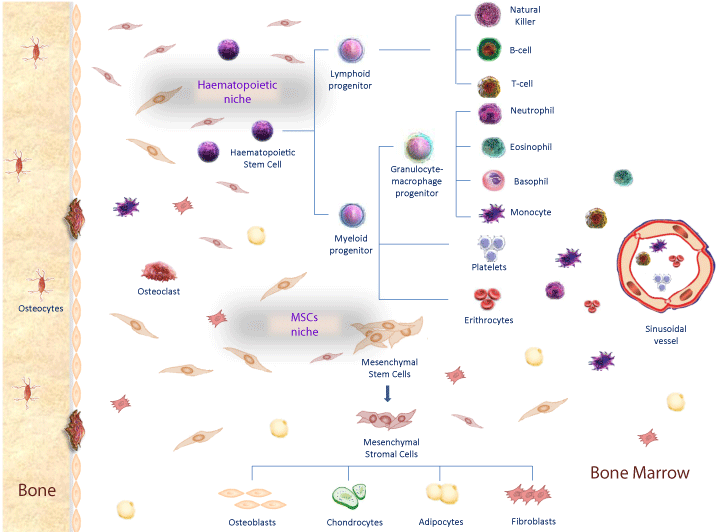
Figure 1: Bone marrow niches and stem cells differentiation. In Haematophoietic niche, MSCs contribute to the creation of microenvironment that promote haematopoietic stem cells differentiation in myeloid and lymphoid progenitors, that differentiate in several blood components such as lymphocytes, granulocytes, platelets and eritrocytes. In Mesenchymal Stem Cell niche, MSCs differentiate in mesenchymal stromal cells and then in osteoblasts, condrocytes, adypocites and fibroblasts in response to different transcription factors.
Mesenchymal Stromal Cells in Solid Tumor
Recent studies showed that MSCs are involved in tumorigenesis, being able to integrate into solid tumors [31,32]. Marrow stroma formation is essential for tumor growth and requires the interaction between malignant tumor cells and non-malignant stromal cells [31,32]. Mesenchymal stromal cells represent the neoplastic component of different bone sarcomas: Osteosarcoma, Chondrosarcoma and Giant Cell Tumor of Bone) (Figure 2).
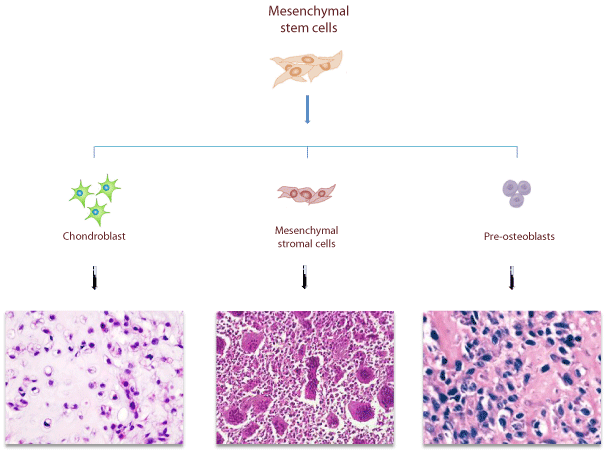
Figure 2: Mesenchymal origin of different bone sarcomas. Mesenchymal origin of different bone sarcomas deriving from different committed stromalrelated cells: Osteosarcoma, derives from uncontrolled proliferation of MSCs committed to osteoblastic lineage; Giant Cell Tumor of bone is caused by neoplastic proliferation of immature mesenchymal stromal cells; Chondrosarcoma, derives from uncontrolled proliferation of MSCs committed to chondroblastic lineage.
Osteosarcoma
Osteosarcoma (OS) is the most common primary solid malignant tumor of the bone that occurs in the metaphyseal regions of long bones, mainly in young patients [33]. Almost constantly intramedullary, it may rarely originate at the bone surface. OS shows high tendency and resistance to conventional chemotherapeutic treatment and the majority of secondary recurrences are due to pulmonary metastasis [34]. This bone sarcoma is a consequence of genetic and epigenetic alterations in mesenchymal progenitor cells committed toward osteoblastic lineage that produce osteoid and/or immature bone resulting in sarcomatous degeneration [35]. Several studies hypothesized that this tumor could derive from less mature precursors of osteoblast cells because these cells are able to differentiate in chondroblastic, fibroblastic and osteoblastic components [36]. Germline mutations in retinoblastoma (Rb) and in Tumor suppressor p53 (TP53) genes are associated with OS development. Other genes are probably involved, but the high rate of genetic instability that characterizes this tumor complicates the identification of the causative gene. Interestingly, OS patients show aberrant activation of the Wnt signaling due to an accumulation of β-catenin in the cytoplasm or in the nucleus [37]. Therefore, Wnt signaling hyper activation enhances the expression of c-myc oncogene, responsible for neoplastic proliferation of OS cells, and of several MMPs (MMP-9 and MMP-14), responsible for OS metastatic invasion and associated with poor disease survival [38]. The mesenchymal nature of OS cells is also supported by GLI2 over expression in OS patients, a transcription factor whose overexpression enhances mesenchymal stem cells proliferation and accelerates cell cycle progression [39-41].
Giant Cell Tumor of Bone
Giant Cell Tumor of Bone is an aggressive osteolytic bone neoplasm composed of three major cell types: mesenchymal stromal cells, mononuclear (CD68 positive) histiocytic cells and multinucleated osteoclast-like giant cells [42]. Although this tumor mainly arises in the epiphyses of long bones of the appendicular skeleton, it can also occur in other areas [43]. Histologically, GCT lesion is made up of several multinucleated giant cells that are uniformly distributed among mononuclear spindle-like stromal cells and monocytes. Particularly, mononuclear hystiocitic cells (MNHC) and multinucleated giant cells are considered to belong to the monocytic-histiocytic system, because both cell lines express CD68 antigen. It is widely accepted that MNHC and MNGC are secondarily recruited and do not constitute the neoplastic cell population. In fact, the neoplastic component of the tumor is represented by spindlelike stromal cells, which are able to proliferate in vitro and to form tumors in mice [44]. A recent study definitively demonstrated that MSCs represent the neoplastic component of GCT because only stromal cell compartment (CD51-/CD61-/CD14-) show somatic mutations (G34W or G34L) in H3F3A gene, which is mutated in 92% of conventional GCT patients [45]. Conversely, H3F3A mutations are not detected in giant cell osteoclasts-like (CD51+/CD61+), that represent the osteolytic component of GCT. Giant cells derive from hematopoietic precursors and their formation is directed by the stromal cells that produce several chemokines, including stromal cell-derived factor-1 (SDF-1) and monocyte chemoattractant protein-1 (MCP-1), that recruits monocytes to the tumor site [46,47]. Besides, stromal cells also produce macrophage colony-stimulating factor (M-CSF) that is responsible for monocytes proliferation and differentiation [48]. M-CSF also induces RANK expression on monocytes [49]. The mechanism by which H3F3A mutations drive GCT onset is unknown but it can be hypothesized that histone 3.3 mutations directly lead to the alteration of expression of FGF and/ or Wnt signaling. Recent studies demonstrated that GCT patients showed high expression of FGFR2IIIc and TWIST1, two osteogenic markers that regulate MSCs terminal osteoblast differentiation [50- 52]. Indeed, the mechanism through which FGFR2-IIIc contributes to MSCs uncontrolled proliferation remains unclear; it could be speculated that high levels of FGFR2IIIc should promote MSCs osteoblastic differentiation through Runx2 expression. TWIST1 high levels inhibit Runx2 expression and cause the maintenance of mesenchymal stromal cells in immature state. The high recurrence GCT rate upon surgical removal may result from residual stromal cells that are capable of re-forming the tumor that expresses Stro-1 was reported to have stem-like properties [53]. Again, the neoplastic role of the mesenchymal component is supported by the identification that MMP-2 and MMP-9 are expressed in the stromal cells [21]. Microarray analysis confirmed MMP-9 high expression in whole GCT tumor samples and in stromal cells [54,55]. In conclusion, GCT is bone sarcomas whose genetic lesion has recently been identified arising as a result of the uncontrolled proliferation of MSCs.
Chondrosarcoma
Conventional chondrosarcomas represent about 90% of all chondrosarcomas and are divided according to their location in primary and secondary. The majority is primary and arises in intramedullary cavity of bone and is classified into three grades of malignancy (from I to III) [56,57]. Less differentiated chondrosarcomas cells, show more similarity with MSCs, while more differentiated chondrosarcomas share similarities with fully differentiated chondrocytes [58]. Histological and immunohistochemical analyses reveal that chondrosarcoma consists of cells that are in a different differentiation state [59]. The neoplastic component of chondrosarcoma is unknown and no evidence of neoplastic degeneration of adult chondrocytes has been reported. However, the mesenchymal nature seems evident especially for grade III chondrosarcomas, because in its muco-myxoid matrix the cells at the periphery of the lobules may become spindle-shaped, resembling a less differentiated phenotype [60]. Therefore, although the mesenchymal nature of this extremely heterogeneous tumor remains dubious, there is a growing body of evidence identifying it as an additional tumor that results from uncontrolled proliferation of mesenchymal cells that are committed toward chondrocyte line.
Mesenchymal Stromal Cells in Cancer Therapy and Other Clinical Conditions
MSCs have generated a great interest in oncology for their ability to repair which makes them particularly suitable in cell-based therapies [61]. Moreover, because of their remarkable capacity to be recruited from bone marrow into the blood circulation and then into damaged sites, MSCs could be used as vehicles for anti-cancer drugs [62,63]. After intravenous infusion, MSCs accumulate in liver cancerderived structures as well as in tumor stroma in breast cancer and osteosarcoma, while in hematological malignancies MSCs autologous transplantation improves hematopoietic stem cells engraftment in bone marrow [63-66]. Tumor-tropic migratory properties of MSCs derive from stimuli produced by the tumor tissue (chemokines) and from their intrinsic properties (chemokine receptors) [67]. For this reason, MSCs have also been used as vehicles to efficiently deliver oncolytic viruses into tumors and metastatic sites in models of breast carcinoma, ovarian cancer and glioma [68-71]. The tumorsuppressive effects of MSCs are due to the down-regulation, through Wnt inhibitors, of Wnt signaling target genes that are involved in anti-apoptosis, cell proliferation and cell cycle regulation [72,73]. This effect could also derive from their ability to inhibit NF-kB pathway in cancer cells and to produce tumor necrosis factor-related apoptosisinducing ligand (TRAIL), inhibiting different type of tumor growth [74,75]. Therefore, the mechanisms of MSCs-based cancer therapy are not yet completely clear and further studies are required to ensure the quality and bio safety of MSCs. MSCs properties also represent an important bio-resource for novel cell and gene-based therapeutic strategy of several non-tumoral conditions. Their capacity to regenerate mesenchymal tissues strongly supports their use in regenerative medicine to replace or repair damaged tissues of mesenchymal origin [76]. Moreover, for their ability to transdifferentiate into cell lineages belonging to not mesoderm embryonic layers (e.g. neurons, liver, kidney and spleen), MSCs represent a useful tool for the treatment of several medical conditions including stroke, spinal cord injuries, acute kidney failure or act as multidrug dispenser to favour tissue regeneration [77,78]. The driving force of MSCs use derives from their role in immune response modulation as they show low inherent immunogenicity, which allows their use for both autologous and allogeneic cell therapies. The non-immunogenic property of MSCs is due to the absence of the expression of class II MHC molecules on their surface that render them able to inhibit T cells activation [79,80].
Conclusion
Studies focused on mesenchymal stromal cells are important to accumulate evidence and information to define their nature for therapeutic approaches. Moreover, this type of information seems crucial to prevent neoplastic degeneration of their committed cell or at least to design better therapeutic approaches for affected patients.
Acknowledgment
We thank Dr Monica Autiero for helpful suggestions and manuscript editing.
References
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007; 131: 324-336.
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466: 829-834.
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495: 231-235.
- Morrison SJ, Scadden DT2. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505: 327-334.
- Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006; 116: 1195-1201.
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001; 414: 98-104.
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002; 3: 931-940.
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008; 135: 1118-1129.
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004; 116: 769-778.
- Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003; 425: 778-779.
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006; 311: 1880-1885.
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008; 9: 11-21.
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007; 25: 238-243.
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006; 7: 333-337.
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006; 6: 93-106.
- Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013; 786: 213-229.
- Zaher W, Harkness L, Jafari A, Kassem M. An update of human mesenchymal stem cell biology and their clinical uses. Arch Toxicol. 2014; 88: 1069-1082.
- McMahon LA, Reid AJ, Campbell VA, Prendergast PJ. Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen-GAG scaffold: experimental and computational analysis. Ann Biomed Eng. 2008; 36: 185-194.
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006; 107: 1878-1887.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282: 1145-1147.
- Ghert M, Simunovic N, Cowan RW, Colterjohn N, Singh G. Properties of the stromal cell in giant cell tumor of bone. Clin Orthop Relat Res. 2007; 459: 8-13.
- Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006; 24: 2412-2419.
- Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004; 16: 681-687.
- Debiais F, Hott M, Graulet AM, Marie PJ. The effects of fibroblast growth factor-2 on human neonatal calvaria osteoblastic cells are differentiation stage specific. J Bone Miner Res. 1998; 13: 645-654.
- Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000; 149: 1297-1308.
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005; 16: 205-213.
- Singh S, Singh M, Mak IW, Turcotte R, Ghert M. Investigation of FGFR2-IIIC signaling via FGF-2 ligand for advancing GCT stromal cell differentiation. PLoS One. 2012; 7: e46769.
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004; 93: 1210-1230.
- Baldini N, Scotlandi K, Barbanti-Bròdano G, Manara MC, Maurici D, Bacci G, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995; 333: 1380-1385.
- Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem. 2006; 281: 1381-1388.
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002; 62: 3603-3608.
- Marini F, Hall B, Dembinski JL, Studeny M, Sasser AK, Andreeff M. Mesenchymal stem cells as vehicles for genetic targeting of tumors. In: Ho, AD, Hoffman R, Zanjani ED, editors. Stem Cell Transplantation. Wiley-VCH Verlag; Weinheim, Germany. 2006; 157-175.
- Bielack S, Carrle D, Casali PG; ESMO Guidelines Working Group. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009; 20 Suppl 4: 137-139.
- Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003; 21: 2011-2018.
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21 Suppl 7: vii320-325.
- Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995; 75: 203-210.
- Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, et al. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002; 102: 338-342.
- Uchibori M, Nishida Y, Nagasaka T, Yamada Y, Nakanishi K, Ishiguro N. Increased expression of membrane-type matrix metalloproteinase-1 is correlated with poor prognosis in patients with osteosarcoma. Int J Oncol. 2006; 28: 33-42.
- Nagao H, Ijiri K, Hirotsu M, Ishidou Y, Yamamoto T, Nagano S, et al. Role of GLI2 in the growth of human osteosarcoma. J Pathol. 2011; 224: 169-179.
- Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009; 152: 15-32.
- Fox MG, Trotta BM. Osteosarcoma: review of the various types with emphasis on recent advancements in imaging. Semin Musculoskelet Radiol. 2013; 17: 123-136.
- Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, et al. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005; 23: 203-209.
- Unni KK, Inwards CY. Giant Cell tumor (osteoclastoma). Dahlin's bone tumors: general aspects and data on 10.165 cases. New York: Lippincott Williams & Wilkins. 2010; 225-242.
- Wülling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003; 34: 983-993.
- Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013; 45: 1479-1482.
- Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, et al. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005; 23: 203-209.
- Zheng MH, Fan Y, Smith A, Wysocki S, Papadimitriou JM, Wood DJ. Gene expression of monocyte chemoattractant protein-1 in giant cell tumors of bone osteoclastoma: possible involvement in CD68+ macrophage-like cell migration. J Cell Biochem. 1998; 70: 121-129.
- Miyamoto N, Higuchi Y, Tajima M, Ito M, Tsurudome M, Nishio M, et al. Spindle-shaped cells derived from giant-cell tumor of bone support differentiation of blood monocytes to osteoclast-like cells. J Orthop Res. 2000; 18: 647-654.
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999; 190: 1741-1754.
- Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002; 277: 36181-36187.
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, et al. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004; 6: 423-435.
- Singh S, Mak IW, Cowan RW, Turcotte R, Singh G, Ghert M. The role of TWIST as a regulator in giant cell tumor of bone. J Cell Biochem. 2011; 112: 2287-2295.
- Lan J1, Liu X, Rong W, Wei F, Jiang L, Yu H, et al. Stro-1(+) stromal cells have stem-like features in giant cell tumor of bone. J Surg Oncol. 2012; 106: 826-836.
- Cowan RW, Mak IW, Colterjohn N, Singh G, Ghert M. Collagenase expression and activity in the stromal cells from giant cell tumour of bone. Bone. 2009; 44: 865-871.
- Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Gene expression in giant-cell tumors. J Lab Clin Med. 2004; 144: 193-200.
- Bertoni F, Bacchini P. Classification of bone tumors. Eur J Radiol. 1998; 27 Suppl 1: S74-76.
- Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977; 40: 818-831.
- Boeuf S, Kunz P, Hennig T, Lehner B, Hogendoorn P, Bovee J, et al. A chondrogenic gene expression signature in mesenchymal stem cells is a classifier of conventional central chondrosarcoma. J Pathol. 2008; 216: 158-166.
- Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005; 6: 599-607.
- Briccoli A, De Paolis M, Campanacci L, Mercuri M, Bertoni F, Lari S, et al. Chondrosarcoma of the chest wall: a clinical analysis. Surg Today. 2002; 32: 291-296.
- Rodríguez R, García-Castro J, Trigueros C, García Arranz M, Menendez P. Multipotent mesenchymal stromal cells: clinical applications and cancer modeling. Adv Exp Med Biol. 2012; 741: 187-205.
- Gao Z, Zhang L, Hu J, Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine. 2013; 9: 174-184.
- Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011; 8: 1538-48.
- Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal stem cells for clinical application. Vox Sang. 2010; 98: 93-107.
- Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007; 13: 5020-5027.
- Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009; 281: 32-41.
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007; 13: 72-81.
- Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007; 105: 157-167.
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006; 5: 755-766.
- Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008; 26: 831-841.
- Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009; 69: 8932-8940.
- Abdel aziz MT, El Asmar MF, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, et al. Efficacy of mesenchymal stem cells in suppression of hepatocarcinorigenesis in rats: possible role of Wnt signaling. J Exp Clin Cancer Res. 2011; 30: 49.
- Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J, et al. Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumour Biol. 2014; 35: 1239-1250.
- Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing lig and delivery for cancer therapy. Cancer Res. 2010; 70: 3718-3729.
- Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD. NF-kappaB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin. 2008; 29: 333-340.
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999; 5: 309-313.
- Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004; 8: 301-316.
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011; 9: 11-15.
- García-Castro J, Trigueros C, Madrenas J, Perez-Simòn JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008; 12: 2552-2565.
- Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, et al. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008; 180: 1598-608.