
Research Article
J Blood Disord. 2014;1(4): 3.
Apoptosis of Human Multidrug-Resistant Myeloid Leukemia (HL60/AR) Cells by Thymax Plus Lactobacillus Kefiri P-IF★
Ghoneum M1*, Henary M1 and Seto Y2
1Department of Otolaryngology, Charles Drew University of Medicine and Science, USA
2Tamachi IC Clinic, University of Tokyo, Japan
*Corresponding author: Ghoneum M, Department of Otolaryngology, Charles R. Drew University of Medicine and Science, 1621 E. 120th Street, Los Angeles, California 90059, USA.
Received: October 18, 2014; Accepted: November 08, 2014; Published: November 12, 2014
Abstract
We have recently reported the susceptibility of human Multidrug-Resistant (MDR) myeloid leukemia (HL60/AR) cells to the apoptotic effect of L. kefiri P-IF, a freeze-dried form of heat-killed Lactobacillus kefiri. In this study, we evaluated the synergizing apoptotic effects of L. kefiri P-IF in the presence of Thymax, a gross thymic extract, on HL60/AR cells. To identify any synergistic effect of these two agents, tumor cells were cultured for three days with 0.6-5.0 mg/ ml L. kefiri P-IF alone, 0.6-5.0 mg/ml Thymax alone, or a combination of both agents. The apoptotic response was assessed using a propidium iodide assay. The expression of Bcl-2, an anti-apoptotic protein, was determined by flow cytometry. Results showed that L. kefiri P-IF and Thymax induced apoptosis in HL60/AR cells in a dose dependent manner that was detected at 0.6 mg/mL and maximized at 5 mg/mL. However, treatment by L. kefiri P-IFplus Thymax synergistically induced higher levels of apoptosis in cancer cells that exceeds the effect of either agent alone. The synergistic apoptotic effect was associated with decreased expression of Bcl-2. This combination may represent a new class of adjuvant for the treatment of myeloid leukemia.
Keywords: Thymax; Lactobacillus kefiri P-IF; Apoptosis; Leukemia; Synergy
Introduction
Acute Myeloid Leukemia (AML) is a clonal disease characterized by the proliferation and accumulation of myeloid progenitor cells in the bone marrow, which ultimately leads to hematopoietic failure [1]. The American Cancer Society estimates that there will be approximately 19000 new cases of AML in the United States for 2014. The successful treatment of many types of cancers has been severely limited by the resistance of tumors to chemotherapeutic agents known as the Multidrug Resistance (MDR). MDR in leukemia has been the focus of research in the last few decades, particularly in acute myeloid leukemia. Several drug resistance proteins that pump out many anti-leukemic agents from cells have been implicated in MDR, including the MDR protein MDR1 (p-glycoprotein), the MDR-associated protein (MRP), and the Lung Resistance Protein (LRP) [2,3]. Intensive chemotherapy, including combinations of high-dose AraC and anthracyclines, are the mainstay of AML patient therapy [4]. Additionally, there has also been studies of Gemtuzumab Ozogamicin (GO) used for AML treatment; GO is a conjugate of a cytotoxic agent, a calicheamicin derivative. However, more than 50% of patients are expected to relapse following intensive chemotherapy, which is often associated with clinical drug resistance [4,5]. The mechanism of P-glycoprotein (P-gp), a MDR trans-membrane glycoprotein, also affects GO. Therefore, we thought it would be of particular interest to find new agents that are non-toxic and possess the ability to cause apoptosis in MDR leukemic cells.
We recently reported the apoptotic effect of a novel kefir product, L. kefiri P-IF, on MDR myeloid leukemia cells via a hole-piercing mechanism [1]. The current study was carried out to examine the synergizing effect of L. kefiri P-IF in the presence of another apoptotic agent namely Thymax, a gross thymic extract, in inducing apoptosis in MDR myeloid leukemia cells in vitro. This combination may represent a new class of adjuvant for the treatment of myeloid leukemia.
Materials and Methods
Tumor cell line and culture conditions
.Human MDR myeloid leukemia (HL60/AR) cells were used in the present study. Cells were kindly provided by Dr. S. Gollapudi at the University of California, Irvine. Tumor cells were maintained in our laboratory in a complete medium (CM) that consisted of RPMI-1640, supplemented with 10 percent fetal calf serum, 2 mM glutamine, and 100 μg/ml streptomycin and penicillin.
Drugs and chemicals
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma-Aldrich, (St. Louis, MO, USA).
Thymax is a gross thymic extract obtained at acidic pH (through treatment with NaCl and L-ascorbic acid) as described [6]. Thymax was dissolved in Distilled Water (DW) at concentration of 1gm/ml. Thymax was provided by the YS Nature Company, Tokyo, Japan
PFT is a mixture that mainly (~90%) contains a freeze-dried form of heat-killed L.kefiri P-IF; it is a specific strain of LAB that has a unique DNA sequence and PET scans show a 99.6% homology with regular kefiries. PFT also contains ~2-3% each of bacterial strain, Lactobacillus kefiri P-IF and Lactobacillus kefiri P-B1 and three yeast strains, Kazachstania turicensis, Kazachstania unispora and Kluyveromyces marxianus [7]. PFT was dissolved in Distilled Water (DW) at concentration of 50 mg/ml. PFT was provided by Paitos Co., Ltd. Yokohama, Kanagawa, Japan.
Probiotics fermentation technology (PFT) kefir grain product
HL60/AR cells were cultured for three days with Thymax at a different concentrations (0.6, 1.25, 2.5 and 5 mg/ml) or L. kefiri P-IF (0.6, 1.25, 2.5 and 5 mg/ml) or a combination of both agents at the same concentrations. Results were compared to cancer cells without any treatment. The percentage of dead cancer cells was examined by the Propidium Iodide (PI) technique using a FACS calibur flow cytometery. In this technique, dead cells pick up PI and fluoresce. Briefly, PI was added to the cells (1x106/ml) to give a final PI concentration of 50 μg/ml. The cells were stained for 30 min at room temperature in the dark and analyzed by FACS calibur (Becton- Dickinson, San Jose, CA, USA).
Detection of cancer cell viability using propidium iodide
HL60/AR cells were cultured for three days with Thymax at a different concentrations (0.6, 1.25, 2.5 and 5 mg/ml) or L. kefiri P-IF (0.6, 1.25, 2.5 and 5 mg/ml) or a combination of both agents at the same concentrations. Results were compared to cancer cells without any treatment. The percentage of dead cancer cells was examined by the Propidium Iodide (PI) technique using a FACS calibur flow cytometery. In this technique, dead cells pick up PI and fluoresce. Briefly, PI was added to the cells (1x106/ml) to give a final PI concentration of 50 μg/ml. The cells were stained for 30 min at room temperature in the dark and analyzed by FACS calibur (Becton- Dickinson, San Jose, CA, USA).
Expression of Bcl-2
For detection of Bcl-2, HL60/AR cells were cultured with Thymax (1.25 mg/ml), or L. kefiri P-IF (1.25 mg/ml) or a combination of both agents at the same concentration. Cells were then fixed and permeabilized with ice-cold 70% methanol and stained with FITClabeled anti-Bcl-2 or isotope control (Dako Corp., Carpinteria, CA, USA). Cells were washed and analyzed by FACS calibur. The percentages of cells expressing Bcl-2 and mean fluorescent intensity (an indicator of density of the molecules/cell) were determined.
Treatment
MFC#
D-value
None
74
Thymax
68
> 0.15
L. kefiri P-IF
61
> 0.28
Thymax + L.kefiri P-IF
50
> 0.31
Table 1: Effect of Thymax, L. kefiri P-IF, and Thymax + L.kefiri P-IF on the expression of Bcl-2. All agents were used at concentration of 1.25 mg/mL. MFC# = mean fluorescence channel number. A D-value > 0.15 is considered statistically significant.
Statistical analysis
Statistical significance for cell apoptosis in Figure 1 was determined by Student's t test. Differences were considered significant at the p < 0.05 level. Statistical analysis for flow cytometry was performed by the Kolmogorov-Smirnov test using Cell Quest Software system. A D-value of > 0.15 was considered statistically significant.
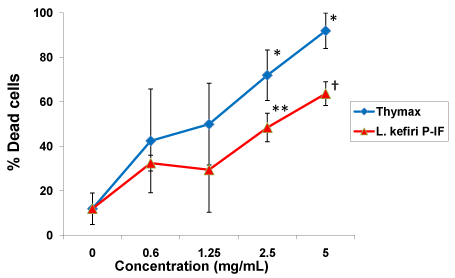
Figure 1: Apoptotic effect of L. kefiri P-IF alone or Thymax alone on HL60/ AR cells. HL60/AR cells were cultured for three days with either L. kefiri P-IF or Thymax at different concentrations (0.6, 1.25, 2.5 and 5 mg/ml). The apoptotic response on HL60/AR cell survival was assessed by using PI technique using a FACS calibur flow cytometry. Data represent the mean ± SD of 3 experiments. Statistical analysis compared the concentrations to control (0 mg/ml):*p<0.02, **p<0.01, †p<0.004.
Results
Apoptotic effect of L. kefiri P-IF alone or thymax alone on HL60/AR cells
The apoptotic response on HL60/AR cell survival post treatment with L. kefiri P-IF alone or Thymax alone was assessed by using PI and FACS calibur flow cytometry. Both agents were used at the same concentration (0.6, 1.25, 2.5 and 5 mg/ml). Figure 1 shows Thymax induced an apoptotic effect on HL60/AR cells in a dose dependent manner. The apoptotic effect was detected at concentration of 0.6 mg/ml and maximized at 5 mg/ml (p<0.05). Similarly, L. kefiri P-IF induced an apoptotic effect on cancer cells at concentration of 0.6 mg/ml and further increased at concentration of 5 mg/ml (p<0.05).
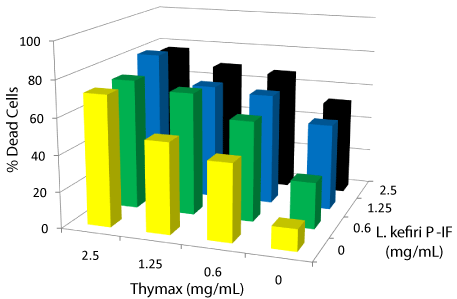
Figure 2: Apoptotic effect of L. kefiri P-IF plus Thymax on HL60/AR cells. HL60/AR cells were treated for three days with L. kefiri P-IF plus Thymax at different concentrations (0.6, 1.25 and 2.5 mg/ml). The apoptotic response on HL60/AR cell survival post treatment with both agents was assessed by using PI technique using a FACS calibur flow cytometry. Data represent the mean of 3 experiments.
Apoptotic effect of L. kefiri P-IF in the presence of thymax at different concentrations
Data in Figure 2 summarizes the results of the apoptotic effect on tumor cell survival by L. kefiri P-IF plus Thymax at different concentrations of each (0.6, 1.25 and 2.5 mg/ml).The data show a synergistic apoptotic effect at all concentrations used, and this effect increased in a dose-dependent manner as the concentration increased up to 2.5mg. It is found that the highest synergistic apoptotic response is reached with Tymax at 2.5mg/mL and L. kefiri P-IF at 1.25 mg/mL concentrations. In addition, the level of synergy exceeds the effect of either agent alone.
Expression of Bcl-2
Bcl-2 is an anti-apoptotic protein. Expression of Bcl-2 was determined by flow cytometry. HL60/AR cancer cells (1 x 106 cells/ ml) were cultured for 24 hours with Thymax (1.25 mg/mL), L. kefiri P-IF (1.25 mg/ml), or Thymax + L.kefiri P-IF(1.25 mg/ml). Expression of Bcl-2 was determined by staining the cells with anti-human Bcl-2 antibody and flow cytometry (MFC# = mean fluorescence channel number). Results depicted in Table 1 showed that treatment with Thymax and L. kefiri P-IF caused a significant decrease in the expression of Bcl-2 as follows: MFC# was 68 for Thymax and 61 for L. kefiri P-IF. However, a synergistic effect showed a greater decrease of Bcl-2 expression with a MFC# 50 when cancer cells were cultured in the presence of both Thymax and L. kefiri P-IF as compared to 74 for control cancer cells without treatment.
Discussion
Data of the current study show susceptibility of the MDR myeloid leukemia (HL60/AR) cells to the apoptotic effect of L. kefiri P-IF plus Thymax, a gross thymic extract. Probiotics Fermentation Technology (PFT) kefir grain product is a mixture whose the main constituent is the L. kefiri P-IF strain. It has a unique DNA sequence and shows a 99.6% homology with regular kefiries and similar 16S ribosome sequence compared with other L.kefiri strains [7]. We have recently demonstrated the ability of L. kefiri P-IF to induce apoptosis on HL60/AR cells [1]. The mechanism underlying the apoptotic effect by L. kefiri P-IF may involve piercing holes in the cellular membrane of HL60/AR cells. Treatment with L.kefiri P-IF caused 2.6-fold increase in the percentage of cells with holes as compared to control untreated HL60/AR cells [1]. Other mechanisms that may contribute to the anticancer activity by P-IF are the abilities of this agent to utilize galactose as a carbon source, and produce carbonic acid upon agitation of its growth medium. In addition, it grows threedimensionally, which is attributed to the unique carbohydrate chains on its surface [7]. These characteristics make P-IF a unique, effective anticancer agent as compared with other L.kefiri strains.
Thymax, a gross thymic extract, is an immune-activating compound that has the ability to activate human Dendritic Cells (DC) and the DC-directed T-cell response in an in vitro culture model [8]. Furthermore, Thymax induces a reversal of age-associated decline in the function of murine immune cells [9]. Further studies showed that Thymax is an apoptotic agent in human breast cancer cells. It exerts its effect via the mitochondrial pathway as indicated by the activation of capsizes 8 and 9 and the significant decrease in the Mitochondrial Membrane Potential (MMP) [6]. Results of the current study also show that Thymax exerts a potent apoptotic effect on HL60/AR cells.
The mechanism by which L.kefiri P-IF and Thymax induced apoptosis in HL60/AR cells was examined. The apoptotic effect by these agents on HL60/AR cells was associated with a decrease in the protein expression of Bcl-2. The mitochondrial pathway is mainly governed by the Bcl-2 family of proteins, which include pro-apoptotic (Bax, Bad, Bid, etc.) and anti-apoptotic (Bcl-2, Bcl-XL) proteins. The results of the current study indicated a down-regulation of Bcl-2 by either L kefiri P-IF or Thymax in HL60/AR cells. Furthermore, Bcl- 2 levels to a greater extent when HL60/AR cells were treated with a combination of the L.kefiri P-IF plus Thymax than either agent alone. Bcl-2 has been shown to play a crucial role in the maintenance of MMP. The permeabilization of the mitochondrial membranes results in the release of pro-apoptotic molecules into the cytosol that through cascades of events that leads to the release of pro-caspase 9, which is dimerized and activated. The active caspase 9 activates executioner caspases in order to orchestrate apoptosis [10-13].
The safety of many anti-cancer drugs is still a major concern. Several chemotherapeutic drugs that are the mainstay of AML patient therapy are known to be toxic. In contrast, L.kefiri P-IF appears to be a safe, nontoxic agent. Mice showed no macroscopic or histopathological abnormalities in different organs post-treatment with L.kefiri P-IF and no changes in their body weight as compared with control untreated mice [14]. Similarly, Thymax was shown to be safe. Results of studies of mice treated with Thymax demonstrated no pathology detected in different organs and normal animal behavior [9].
In conclusion, results of this study showed that a combination of Thymax and L. kefiri P-IF exert a potent apoptotic effect on HL60/AR cells by a mechanism that involves down-regulation in the expression of Bcl-2. These two agents may represent a new class of adjutants that could be used to improve the treatment of MDR leukemia.
Acknowledgement
L. kefiri P-IF was provided by Paitos Co., Ltd. Yokohama, Kanagawa, Japan. Thymax was provided by the YS Nature Company, Tokyo, Japan. This work was supported by Paitos Co., Ltd. Grant #T0099108.
References
- Ghoneum M, Gimzewski J. Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int J Oncol. 2014; 44: 830-837.
- Leith C. Multidrug resistance in leukemia. Curr Opin Hematol. 1998; 5: 287-291.
- Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004; 122: 166-171.
- Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009; 31 Pt 2: 2349-2370.
- Takeshita A. Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol. 2013; 97: 703-716.
- Ghoneum M, Seto Y, Sato S, Ghoneum A, Braga M, Gollapudi S. Gross thymic extract, Thymax, induces apoptosis in human breast cancer cells in vitro through the mitochondrial pathway. Anticancer Res. 2008; 28: 1603-1609.
- Suzuki K, Tani H, Yabumoto T, Yabumoto Y, Yoshida Y. Novel fermented milk product and use thereof. US Patent No. US 20110123640 A1. 2011.
- Ghoneum M, Seto Y, Agrawal S. Activation of human monocyte-derived dendritic cells in vitro by Thymax, a gross thymic extract. Anticancer Res. 2009; 29: 4367-4371.
- Ghoneum M, Tolentino L, Seto Y. Phenotypic correction of Age-associated functional decline in murine immune cells by Thymax, a thymic extract. In Vivo. 2009; 23: 895-902.
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281: 1309-1312.
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998; 281: 1305-1308.
- Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001; 292: 624-626.
- Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005; 121: 671-674.
- Paitos Co., Ltd. Yokohama, Kanagawa, Japan: Increase the good bacteria held by nature, ideal AH21 is a functional food, consider preventive medicine and food. 2013.