
Special Article - Colorectal Cancer
Austin J Cancer Clin Res 2015;2(1): 1023.
Optimal Morphologic Response to Chemotherapy and Bevacizumab Correlates with Long Term Outcome in a Patient with Colorectal Liver Metastatic Cancer
Areses C1* and Salgado M2
1Deparment of Medical Oncology, Complejo Hospitalario Universitario de Ourense, Spain
2Deparment of Medical Oncology, Complejo Hospitalario Universitario de Ourense, Spain
*Corresponding author: Areses C, Department of Medical Oncology, Complejo Hospitalario Universitario de Ourense, Ramón Puga 54-56, 32005, Ourense, Spain.
Received: November 26, 2014; Accepted: January 10, 2015; Published: February 16, 2015
Abstract
Since the introduction of antiangiogenic agents, RECIST criteria sometimes are insufficient in assessing response to chemotherapy in colorectal liver metastases (CLM). According to CHOI criteria it has been seen that morphologic changes in tumor can predict pathologic response and long-term survival of CLM patients treated with chemotherapy, with or without bevacizumab in resectable or unresectable disease.
We present a case of colorectal liver metastases (CLM) treated with bevacizumab-containing chemotherapy that obtained an optimal morphologic response and long-term outcome.
Keywords: Colorectal liver metastases; Bevacizumab; Pathologic response; Morphologic response; Overall survival
Case Presentation
The patient is a smoker 54-year-old woman who initially presented to her primary care provider with lower abdominal pain and progressive weight lost for 3 months. There was nothing particular regarding her past medical history or family history.
Initial laboratory workup showed: Hemoglobin (Hbg) 9.5 gr/ dL; Gamma glutamyl transpeptidase (GGT) 229 UI/L; and alkaline phosphatase (FA) 437 UI/L. The serum level of Carcinoembryonic antigen (CEA) was 9119 ng/ml. Abdominal and pelvic ultrasonography showed a heterogenous mass in rectum-sigmoid colon and multiple liver lesions. Colonoscopy revealed a stenosant large mass at 50 cm of the anal edge. Biopsy specimens were histologically identified as adenocarcinoma grade 2.
Chest-abdominal and pelvic computerized tomography (CT) confirmed the presence of a mass nearly obstructing the sigmoid colon and liver metastases, two of them in segments V and VIII and other two in segments IV a VI.
She underwent sigmoidectomy and the patient tolerated the procedure well, except for surgical site infection that was treated with an antibiotic that covered the likely causative organisms.
The pathological diagnosis of surgical specimen was a low-grade (moderately differentiated) adenocarcinoma with clear margins and one of 11 lymph nodes positive for metastatic carcinoma. It was staged as pT3pN1cM1 because of liver metastases according to American Joint Committee on Cancer, seventh Edition (2010). The result from KRAS test was wild-type.
After the surgery, the patient was started on FOLFIRI chemotherapy (irinotecan 180 mg/m2 intravenously (IV), over 90 minutes, with folinic acid 400 mg/m2, over 120 minutes, followed by fluorouracil 400 mg/m2 IV bolus, then fluorouracil 2400 mg/ m2 IV infusion, over 46 hours) in combination with bevacizumab 5 mg/kg every two weeks. Adverse events (AEs) occurring were grade 1 diarrhea, grade 1 mucositis, grade 1 epistaxis and grade 1 neutropenia. The patient tolerated the treatment very well not requiring chemotherapy dose reduction.
She was evaluated with CT every 3 months and after receiving 9 cycles, the CT revealed an increase of 5 cm in the size of the lesion localized in segments V-VIII but a decrease in density and attenuation (she had liver disease progression according to RECIST criteria but response according to CHOI criteria). Although the discordance of the results, she was asymptomatic and the CEA went down to 121 ng/ mL, so the consensus of the tumor board finally decided to continue with the same treatment. In the next CT the liver lesions started to reduce and calcificate in the periphery getting a response not only according to CHOI criteria, also according to RECIST criteria (Figure 1). The CEA level also continued decreasing till it normalized (Figure 2). After 42 cycles of this chemotherapy regimen, she was started on maintenance with bevacizumab 7.5 mg/kg every 21 days. After 6 cycles, she presented grade 1 urine protein and high blood pressure that was controlled with antihypertensive treatment. After 24 months of treatment, restaging CTs continued showing liver disease stabilized and positron emission tomography (PET) was negative. At this moment, after discussing the case, the consensus of the tumor board finally decided to stop the treatment and the patient started the recommended follow-up having a CT scan and CEA test every three months.
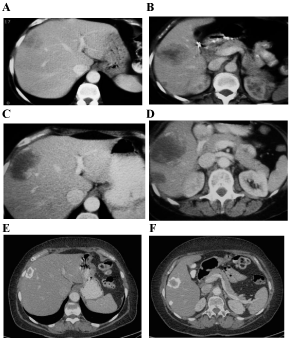
Figure 1: A-B) Liver disease at diagnosis. C-D) After 9 cycles of chemotherapy. E-F) After 42 cycles of chemotherapy.
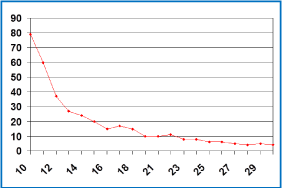
Figure 2: Change in CEA level (ng/mL).
Three years after discontinuing the chemotherapy, she continues to do well with stabilization liver disease and a normal CEA level.
Discussion
Colorectal cancer is the third most common cancer worldwide and accounts for over 1 million new cases of cancer each year. It is the fourth most frequent cause of cancer death worldwide [1].
Surgery can be curative not only in patients with early-stage and locally-advanced colon cancer but also in patients with initially resectable or potentially resectable liver-only metastases [2].
Systemic chemotherapy used before and/or after surgical resection has shown to significantly improve outcomes and achieve long-term survivals [3,4].
The current options for perioperative treatment include the use of 5-fluorouracil (5-FU) based therapies with oxaliplatin [5] or irinotecan [6]. You can also use them in combination with targeted molecular therapies as cetuximab/panitumumab (monoclonal antibodies against epidermal growth factor receptor) [7,8] or bevacizumab (monoclonal antibody that inhibits vascular endothelial growth factor) [9,10].
Rubbia-Brandt et al. [11] and afterwards Blazer et al. [12] demonstrated that pathologic response correlates with overall survival (OS) in patients with CLM treated with preoperative chemotherapy and hepatic resection. In this way, pathologic tumor response to chemotherapy was recognized as an important prognostic factor in patients with CLM treated with preoperative chemotherapy.
Till recently there wasn´t a noninvasive method of predicting pathologic response to chemotherapy in CLM and RECIST criteria sometimes is inadequate in assessing response to chemotherapy, especially in patients treated with a regimen including antiangiogenic agents [13]. In this way Choi et al, reported Choi or morphologic criteria aimed mostly to consider treatment related decrease of tumor density [14].
Chun et al. [15] observed that after bevacizumab-containing therapy, CLM tend not only to decrease in size but also to undergo morphologic changes as decreasing density on CT. In this way, they correlated an optimal morphologic response with a good pathologic response. We already knew about the correlation between pathologic response and OS, so they demonstrated that CLM with optimal morphologic response had a better OS. They confirmed these results not only in patients that receive preoperative chemotherapy but also in patients with unresectable CLM.
Shindoh et al. [16] confirmed that optimal morphologic response is sufficiently correlated with OS to be considered a surrogate therapeutic end point for patients with CLM and it is probably that the integration of morphologic criteria and RECIST may improve the accuracy in prediction of pathologic response and OS [17]. These novel criteria have been recently introduced for patients undergoing chemotherapy for resectable and unresectable CLM.
We report a case of CLM treated with bevacizumab-containing chemotherapy that obtained an optimal morphologic response and liver disease stabilization. At this moment, seven years after the diagnoses she is alive, asymptomatic, without treatment and continuing a close follow up.
Conclusion
An optimal morphologic response to chemotherapy and bevacizumab is correlated with long-term outcomes in CLM. This conclusion is based in the good pathologic response obtained in resected patients and in the long overall survival reached in unresectable patients.
References
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014; 383: 1490-1502.
- Venook AP. The Kemeny Article Reviewed: Management of Liver Metastases from Colorectal Cancer: Review 2. Oncology. 2006; 20.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013; 14: 1208-1215.
- Mitry E, Fields ALA, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant Chemotherapy After Potentially Curative Resection of Metastases From Colorectal Cancer: A Pooled Analysis of Two Randomized Trials. J Clin Oncol. 2008; 26: 4906-4911.
- Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J clin Oncolo. 2005; 23: 9243-9249.
- Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004; 15: 933-939.
- Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadyuvant chemotherapy with cetuximab: the CELIM randomized phase 2 trial. Lancet Oncol. 2010; 11: 38-47.
- Abad A, Massuti B, Grávalos C. Phase II trial of Panitumumab (Pmab) plus FOLFOX4 or FOLFIRI in subjects with KRAS wild-type colorectal cancer and liver limited disease: The PLANET study. WCGI 2014; SEOM. 2014; O3.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350: 2335-2342.
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26: 2013-2019.
- Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007; 18: 299-304.
- Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008; 26: 5344-5351.
- Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014; 19: 394-402.
- Choi H, Charnsangavej C, Faria SC. Correlation of computed tomography and positron emission tomography in patients with metastasic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin Oncol. 2007; 25: 1753-1759.
- Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009; 302: 2338-2344.
- Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, et al. Optimal morphologic response to preoperative chemotherapy: An alternative outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012; 30: 4566-4577.
- Shindoh J, Chun YS, Loyer EM. Non-size-based response criteria to preoperative chemotherapy in patients with colorectal liver metastases: the morphologic response criteria. Curr Colorectal Cancer Rep. 2013; 9: 198–202.