
Special Article – Breast Cancer- Risk Factors, Diagnosis and Treatment
Austin J Cancer Clin Res 2015;2(1): 1024.
The Benefit of 18f-FDG PET/CT in Breast Cancer Patient Staging and Treatment Planning: A Case Report
Valli M*, Belosi MF, Nicolini G and Richetti A
Radiation Oncology, Oncology Institute of Southern Switzerland, Switzerland
*Corresponding author: Valli M, Radiation Oncology Unit, Oncology Institute of Southern Switzerland, Via Ospedale, 6500 Bellinzona (CH), Switzerland.
Received: January 30, 2015; Accepted: February 18, 2015; Published: February 20, 2015
Abstract
A 50 year old female patient was treated with left mastectomy plus axillary dissection for ductal infiltrating carcinoma mpT1c pN1(1/15) M0 in October 2009. The patient was staged post-operatively with 18F-FDG PET/CT and unexpected internal mammary chain lymph node metastases were detected. The patient received adjuvant chemo/hormonal therapy and radiotherapy on regional lymph nodes up to a dose of 50 Gy with a volume modulated arc (VMAT) technique, reaching a complete response. At 5 year follow-up the patient is still alive in complete response without radiotherapy late toxicity.
Keywords: 18F-FDG PET/CT; Breast cancer; Internal mammary chain; VMAT
Introduction
The role of 18F-FDG PET/CT in the staging of breast cancer is still controversial. Its use in early stage cases is not recommended, whereas in locally advanced cases it can lead to a change in the staging phase from 6.7% to 52%and consequently to a change in the treatment approach from 5.6% to 56% of the cases [1,2].
18F-FDG PET/CT improves the diagnosis of undetected lymph node metastases outside the axilla (i.e. infraclavicular, supraclavicular and internal mammary nodes) or occult and asymptomatic distant metastases [3].
Case Presentation
A 50 year old female patient detected a lump in the inner-superior quadrant of left breast. Subsequent mammography, ultrasound and fine needle aspiration confirmed a ductal infiltrating carcinoma and she was treated with mastectomy, simultaneous reconstruction with prosthesis and contralateral mastoplasty. The final diagnosis confirmed a ductal infiltrating carcinoma ER95%, PR80%, Ki67 20%, c-erbB2 score 2 (FISH neg), lymphovascular invasion, mpT1c pN1(1/15) M0 associated to peritumoral DCIS G3 (5-10%). A postoperative 18F-FDG PET/CT was performed and the final stage was modified in pN3b due to the detection of metastases in the internal mammary chain lymph nodes (IMC LN). The diagnose was confirmed by a fine needle aspiration on the largest lymph node. Therefore the patient received 4 adjuvant chemotherapy cycles (AC schedule), LHRH analogues for 2 years, and aromatase inhibitors for 5 years and radiotherapy at the end of chemotherapy.
The radiotherapy treatment was performed with VMAT Rapid Arc (Varian Medical System, Palo Alto, California) from April 2010 until May 2010. The prescribed total dose was of 50 Gy, 5 fractions per week, 2 Gy each. The post-operative 18F-FDG PET/CT was merged with the planning CT. The contoured Clinical target Volume (CTV) included the supraclavicular/infraclavicular lymph nodes and the IMC. The chest wall was excluded due to fact that the tumor size was <2 cm and due to the presence of the prosthesis. The 18F-FDG PET/CT allowed a correct detection of the IMC LN, not visible on the planning CT (Figure 1). The Planning Target Volume (PTV) was obtained extending the CTV up to 0.8 cm and cropped 0.2 cm from the body surface. As Organs at Risk (OARs), the contralateral breast, the ipsilateral prosthesis, the lungs, the spine, the heart, the thyroid and the left anterior descending coronary artery (LAD), were considered. A dedicated structure was contoured for minimizing the dose spillage outside the PTV, named Healthy Tissue (HT). Such structure was drawn at 0.2 cm distance from the PTV and extended up to 2.0 cm. The treatment plan was designed on the Varian Eclipse Treatment Planning System (platform 8.6) for a Varian iX linear accelerator, 6 MV energy beam. Two partial arcs (CW and CCW) were applied in order to avoid beam entrances from the controlateral lung and controlateral breast. Inverse planning was performed by means of the Progressive Resolution Optimizer III, version 10.0.28, employing the intermediate dose options in order to increase the accuracy of optimization and the agreement between optimization and final dose calculation. Dose-volume constraints during the optimization were defined to match the planning goals of having more than 95% of the PTV volume receiving >95% of the prescribed dose (V95%>95%) and of minimizing the dose to the ipsilateral lung (V20Gy<20%), to the heart and the LAD. The obtained dosimetric parameters are listed in Table 1. The patient concluded the scheduled treatment. Orthogonal X-Ray imaging was performed every day for a correct patient set-up.
On the upper corner of the left hand-side a coronal view of the planning CT where no lymph nodes are visible; the same CT plane is shown in the lower corner blended with the 18F-FDG PET which makes the IMC lymph nodes clearly localizable. The median part of the image displays the sagittal and frontal views of the whole planning CT blended with the 18F-FDG PET, with red arrows indicating the IMC lymph nodes. On the right hand-side the 18F-FDG PET alone, with red arrows indicating the same IMC lymph node.
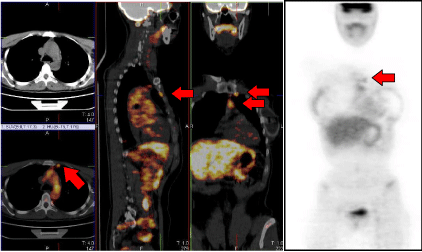
Figure 1: 18F-FDG PET/CT pre-irradiation.
On the upper corner of the left hand-side a coronal view of the planning CT where no lymph nodes are visible; the same CT plane is shown in the lower corner blended with the 18F-FDG PET which makes the IMC lymph nodes clearly localizable. The median part of the image displays the sagittal and frontal views of the whole planning CT blended with the 18F-FDG PET, with red arrows indicating the IMC lymph nodes. On the right hand-side the 18F-FDG PET alone, with red arrows indicating the same IMC lymph node.
Organ
Dosimetric Parameters
PTV
V95% = 95% V107% = 0% D2% = 104%
Ipsilateral lung
Mean Dose = 8.4 Gy V20Gy = 10%
Ipsilateral breast prosthesis
V30% = 12%
Controlateral lung
Mean Dose = 7.4 Gy
Controlateral breast
Mean Dose = 2.7 Gy
Heart
Mean Dose = 8.0 Gy
LAD
Mean Dose = 4.8 Gy D2% = 12.6 Gy
Table 1: Dosimetric parameters. Dosimetric parameters resulting from the treatment planning of the patient for the PTV and OARs. Vx represents the percentage of volume receiving an x% (or x Gy) level of dose; Dy represents the dose received by a percentage y of the volume.
Only grade I cutaneous toxicity (EORTC/RTOG scale) was detected during treatment.
In June 2010 a 18F-FDG PET/CT confirmed the complete response on macroscopic IMC but a mammography detected a doubtful area in the upper external quadrant of the right breast. Since a fine needle aspiration was suspicious for DCIS the patient underwent right mastectomy and simultaneous reconstruction with prosthesis, in July 2010. No cancer cells were found in the mastectomy specimen, while a papilloma associated with ductal hyperplasia was detected.
Up to date the patient has been regularly followed up. No late toxicity was registered. Noteworthy the left prosthesis profile was not altered and the controlateral mastectomy was performed without any complications, despite their inclusion in the irradiated volume.
Discussion
18F-FDG PET/CT was requested, after a multidisciplinary discussion, because of the breast cancer site (inner quadrant). It is known from literature that the risk of IMC metastases is around 3% in upper outer quadrant breast cancer stage I, and >65% in internal inner quadrant in case of positive axillary lymph nodes [4].
This case report strengthened the role of high sensitive and sensible diagnostic tool, such as 18F-FDG PET/CT, in the localization of IMC LN not adequately detectable by other diagnostic approaches. This patient was treated according to the real stage both through systemic and loco-regional therapy.
The IMC localization has always been problematic and controversial due to the lack of a non-invasive accurate diagnostic tool: lymphoscintigraphy and CT (indirect detection) can reach an accuracy of 5 mm and 6 mm in depth, and of 6 mm and 7 mm in lateral direction, respectively; ultrasonography is not accurate enough [5,6]. Consequently in multicentric randomized trials, investigating the role of IMC radiotherapy, no specific recommendations regarding the IMC site has been given. As an example, according to the EORTC 22922/10925 trial [7], the IMC is located in the parasternal region, and approximately 85% of the lymph nodes lie within 4 cm lateral to the midline and within 4 cm depth in the first 3 intercostal spaces. The top of the IMC lies behind the insertion of the sternocleido muscle in the medial supraclavicular area. According to the French trial [8] the IMC is located in the first five intercostal spaces. In the current case the 18F-FDG PET/CT identified the presence of IMC LN even at 5 cm depth and 4.5 cm laterally from the midsternum line (Figure 2), allowing to draw the target volume for irradiation with definitively high confidence. To remark is also the fact that the same imaging conducted in June 2010 revealed a complete response (Figure 3) in contrast with the suspicious mammography. The report of the 18F-FDG PET/CT was then confirmed by pathology specimen. In conclusion, the here reported case is a strong example of the clinical efficacy of 18F-FDG PET/CT for diagnose and staging of IMC LN.
Coronal view of the planning CT blended with the post-operative 18F-FDG PET. In red the PTV volume contoured on the basis of the PET image information. The red line measures the depth of the detected lymph node from the body surface (around 5 cm).
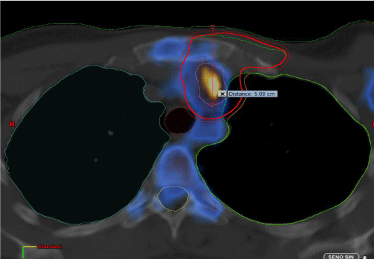
Figure 2: Location of one of the IMC lymph nodes.
Coronal view of the planning CT blended with the post-operative 18F-FDG PET. In red the PTV volume contoured on the basis of the PET image information. The red line measures the depth of the detected lymph node from the body surface (around 5 cm).
In the first raw, three coronal views of the 18F-FDG PET/CT acquired in June 2010 after irradiation; in the second raw, the same slices are shown from the 18F-FDG PET/CT pre-irradiation (December 2009). In the latter images, an up-take of 18F-FDG is clearly visible in correspondence of the IMC lymph nodes, whereas the former images reveal a complete remission of the patient.
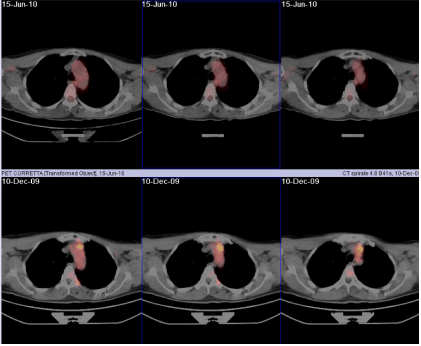
Figure 3: 18F-FDG PET/CT pre-and post-irradiation.
In the first raw, three coronal views of the 18F-FDG PET/CT acquired in June 2010 after irradiation; in the second raw, the same slices are shown from the 18F-FDG PET/CT pre-irradiation (December 2009). In the latter images, an up-take of 18F-FDG is clearly visible in correspondence of the IMC lymph nodes, whereas the former images reveal a complete remission of the patient.
References
- Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013; 54: 5-11.
- Riegger C, Herrmann J, Nagarajah J, Hecktor J, Kuemmel S, Otterbach F, et al. Whole-body FDG PET/CT is more accurate than conventional imaging for staging primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2012; 39: 852-863.
- Gunalp B, Ince S, Karacalioglu AO, Ayan A, Emer O, Alagoz E. Clinical impact of (18)F-FDG PET/CT on initial staging and therapy planning for breast cancer. Exp Ther Med. 2012; 4: 693-698.
- Lievens Y, Van den Bogaert W. Internal mammary and medial supraclavicular lymph node irradiation: the thin line between advantages and side effects. Radiother Oncol. 2002; 65: 75-77.
- Struikmans H, Scheijmans LJ, van Dalen A, Harmelink M, Westers P, van Isselt JW. Lateralisation and depth of the internal mammary chain determined by scintigraphy and by ultrasonography: a comparative study in 124 primary breast cancer patients. Radiother Oncol. 2002; 62: 159-162.
- Saarnak AE, Hurkmans CW, Pieters BR, Valdés Olmos RA, Schultze Kool LJ, Hart AA, et al. Accuracy of internal mammary lymph node localization using lymphoscintigraphy, sonography and CT. Radiother Oncol. 2002; 65: 79-88.
- Poortmans P, Kouloulias V, van Tienhoven G, Collette L, Struikmans H, Venselaar JL, et al. Quality assurance in the EORTC randomized trial 22922/10925 investigating the role of irradiation of the internal mammary and medial supraclavicular lymph node chain works. Strahlenther Onkol. 2006; 182: 576-582.
- Hennequin C, Bossard N, Servagi-Vernat S, Maingon P, Dubois JB, Datchary J, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013; 86: 860-866.