
Editorial
Austin J Cancer Clin Res 2015;2(2): 1028.
Bendamustine and Cytosine Arabinoside: A Highly Synergistic Combination
Visco C*, Carli G and Rodeghiero F
Department of Cell Therapy and Hematology, San Bortolo Hospital, Italy
*Corresponding author: Carlo Visco, Department of Cell Therapy and Hematology, San Bortolo Hospital, Via Rodolfi 37, 36100 Vicenza, Italy.
Received: January 07, 2015; Accepted: March 16, 2015; Published: April 03, 2015
Editorial
Bendamustine is a bifunctional compound that has shown clinical activity against various human cancers including non-Hodgkin’s and Hodgkin’s lymphoma [1,2], chronic lymphocytic leukemia (CLL) [3], multiple myeloma [4,5], breast cancer [6], and small-cell lung cancer [7,8].
Structurally, it comprises two main elements (Figure 1): a 2-chloroethylamine group and a benzimidazole ring. The 2-chloroethylamine alkylating group is shared with other alkylators like cyclophosphamide, chlorambucil and melphalan. Instead, the benzimidazole central ring system is unique to bendamustine and has antimetabolite properties typical of purine analogues. This ring structure may contribute to the peculiar antitumor activity of bendamustine and distinguishes it from conventional alkylators [9]. Both preclinical [10,11] and clinical [12] studies have shown that bendamustine is active in cancer cells that are resistant to conventional alkylating agents.
Figure 1: Chemical structure of bendamustine and cytarabine.
The benzimidazole central ring is likely to be the moiety responsible for its striking effect when combined with cytosine arabinoside (cytarabine). Cytarabine is a pyrimidine analogue of cytidine with an arabinose as sugar moiety instead of ribose (Figure 1). The drug activity depends on the phosphorylation of the prodrug ara-C to the active metabolite Ara-C triphosphate (Ara-CTP) [13,14]. Ara-CTP inhibits DNA polymerase and is incorporated into DNA, ceasing DNA replication during the S-phase of the cell cycle [15-17].
Purine analogues like fludarabine and cladribine are known to augment the intracellular level of ara-CTP, thereby increasing the inhibitory effect of cytarabine on DNA synthesis. Many regimens used for treating acute leukemia’s are based on the association of these drugs [18]. Bendamustine, similarly to nucleoside analogues, has been shown to elicit potent modulation of cytarabine metabolism in leukemic blasts in vitro [19-21] enhancing the apoptotic effect of cytarabine (Figure 2). Hiraoka et al. [21] found that bendamustine up-regulates ENT1, an intracellular nucleoside transporter, thus enhancing the uptake of cytarabine to an extent comparable with the purine analogue fludarabine.
The two agents induce a block of the cell cycle in the S phase (Figure 3) [22], cause cross-links and DNA strand breaks and obstacle further DNA synthesis [24]. The synergistic effect of bendamustine and cytarabine could be related to the individual mechanism of action of the two drugs, whose serial administration would avoid the saturation of the common pathways. Cells escaping the cell cycle arrest induced by bendamustine and trying to repair their damage would be prone to incorporate the metabolite ara-CTP into DNA, as reported by Staib [19] in acute myeloid leukemia cells. Indeed, the sequential treatment with bendamustine followed by cytarabine was proven to be more effective than simultaneous addition of the two drugs (Figure 2) [21-23]. The S phase of the cell cycle is a crucial step of replication in mantle cell lymphoma (MCL) cells, where cyclin D1 overexpression deregulates the cell cycle at the G1/S phase transition, and is likely the engine continuously pushing cells towards S-phase (Figure 3). Indeed, both drugs are known to be particularly active in patients with MCL.
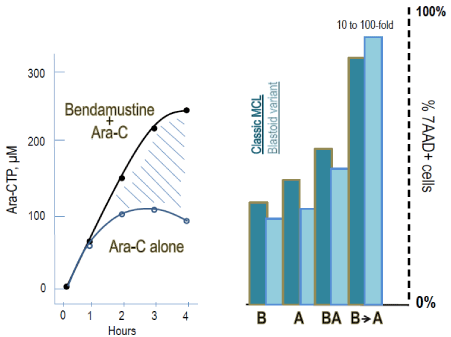
Figure 2: Left: Bendamustine enhances the uptake of cytarabine and
induces increase of Ara-CTP levels in several lymphoma cell lines. Right:
proportion of apoptotic cells measured with 7-AAD following incubation for
24 hours with bendamustine (B) and cytarabine (A) in three condition: drug
alone, simultaneous and consecutive incubation. Histograms refer to mantle
cell lymphoma cell lines, both of the classical and the blastiod form. Data
were extrapolated from Visco et al. [22].
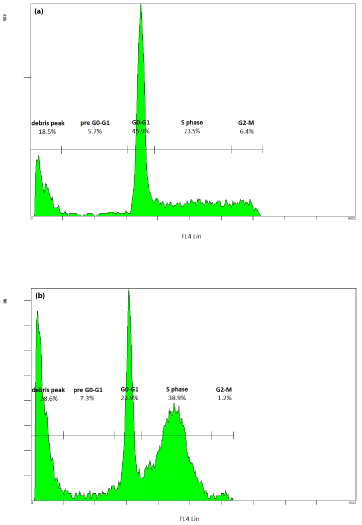
Figure 3: Effect of bendamustine on cell cycle in a mantle cell lymphoma
cell line (JEKO-1) after 24h of incubation: (a) normal cell cycle of JEKO-1
without the drug; (b) incubation with bendamustine induces accumulation of
cells in the S phase and development of a debris peak. Figures reproduced
with permission from Castegnaro et al., and Visco et al. [22,23].
Studies aimed at providing a rationale for the use of this combination in patients with B- and T-cell leukemia/lymphomas or chronic lymphocytic leukemia (CLL) demonstrated that bendamustine and cytarabine are highly synergistic [23]. A relevant and significant improvement of apoptosis when bendamustine and cytarabine were combined in a consecutive manner was observed in CLL cells, either with or without 17p deletion, or resistant to purine analogues [23,24]. Cell lines of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and T-cell lymphomas have also been tested with this combination [23]. A striking synergistic cytotoxic effect was observed in DLBCL, and T-cell lymphoma. Overall, DLBCL and T-cell lymphoma cells were barely affected by the single drugs irrespectively of dose and time of incubation, but both achieved an impressive improvement of cytotoxicity when the drugs were incubated consecutively. The apoptotic rate that characterized DLBCL was more than five-fold greater than the maximum effect obtained with the single drugs [23]. The limited activity of bendamustine as single drug on aggressive lymphoma cell lines resembles what is commonly observed in vivo in clinical trials, where DLBCL and T-cell lymphomas are usually less responsive to the drug than low-grade lymphomas and MCL [25-27].
Two clinical studies have addressed the tolerability and efficacy of the combination of bendamustine and cytarabine, combined with rituximab, in MCL and CLL patients, respectively [28,29]. An open-label, single-arm, phase 2 clinical trial combined the drugs (R-BAC regimen) in MCL patients aged ≥ 65 years who were previously untreated or relapsed/refractory (R/R) to one prior immunochemotherapy [28]. Forty patients (median age 70 years; n = 20 previously untreated) were enrolled. The major toxicity was transient myelosupression, which was significant in the study (87% and 57% of patients with grade 3-4 thrombocytopenia/leukopenia), while febrile neutropenia was quite rare. Non-hematological toxicity was low and 85% of patients, despite the old age, completed at least four cycles without significant clinical complications. The overall response rate (ORR) was 100% (95% complete remission) for previously untreated and 80% (70% complete remission) for R/R patients. After a median follow-up of 26 months (range, 11 to 38 months), the 2-year PFS rate was 95% ± 5% for untreated and 70% ± 10% for R/R patients. A recent update of this trial revealed that, after a median follow-up of 44 months, median PFS has not yet been reached in untreated patients for, while it was 37 months for R/R patients (unpublished report, personal communication). Of note, R-BAC was particularly effective in terms of stem cell harvest, with 100% CD34+ cell mobilizing success in untreated patients. Based on these encouraging results, in order to lower hemato-toxicity, the same authors have initiated a multicenter trial (FIL-RBAC500) promoted by the Fondazione Italiana Linfomi (FIL), with a reduced cytarabine dose of 500 mg/m2 (ClinicalTrials.gov Identifier:NCT01662050). This FIL trial will include minimal residual disease (MRD) evaluations in parallel with PET-scans and will give final results during 2015.
The R-BAC regimen was used for treating patients with R/R CLL in a pilot trial in “ultra-high risk” patients with R/R CLL [29]. All patients had symptomatic disease, were heavily pretreated, had adverse cytogenetics (17p deletion, 11q deletion, or both), and unmutated immunoglobulin heavy chain variable region. Nonhematological toxicity was again acceptable, while major toxicities relied in transient grade 3/4 neutropenia and thrombocytopenia, occurring in 84% and 85% of patients, respectively. Overall, ORR was 84%, including complete and partial response in 38% and 46% of patients, respectively. Patients with 17p deletion had an ORR of 78%. Median progression-free survival was 16 months while median overall survival was not reached (1-year OS 75% ± 13%). Authors concluded that R-BAC is an active regimen in heavily pretreated high risk patients with CLL.
A Phase 2 Study (ClinicalTrials.gov Identifier: NCT01661881) from Dana-Farber Cancer Institute is ongoing for testing rituximabbendamustine followed by rituximab/cytarabine in untreated MCL. Although it is expected that patients will proceed to autologous stem cell transplantation at the conclusion of this regimen, this scheme will give further insights on the efficacy of combining cytarabine with bendamustine in MCL patients.
While several targeted agents are reaching the clinical practice, the association of bendamustine and cytarabine appears one of the most appealing chemo-based combinations in terms of tolerability and efficacy. Since hematological toxicity was relevant, attempts are ongoing in order to lower the impact of the regimen on blood counts while sparing its anti-tumor activity. Further trials are needed to confirm preliminary results in patients with CLL, while pre-clinical data suggest that patients with DLBCL and T-cell lymphomas might benefit of this combination in the near future.
Acknowledgement
TH is work was supported in part by grants from AViLL/AIL (Associazione Vicentina per le Leucemie, i Linfomi e il Mieloma/ Associazione Italiana Leucemie) (Vicenza, Italy) and from VHPF (Vicenza Hematology Project Foundation) (Vicenza, Italy).
References
- Moskowitz A. Bendamustine: a bridge to longer term solutions in heavily treated Hodgkin lymphoma. Leuk Lymphoma. 2013; 54: 2339-2340.
- Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005; 23: 3383-3389.
- Bergmann MA, Goebeler ME, Herold M, Emmerich B, Wilhelm M, Ruelfs C, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica. 2005; 90: 1357-1364.
- Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica. 2005; 90: 1287-1288.
- Pönisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone-a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J Cancer Res Clin Oncol. 2006; 132: 205-212.
- von Minckwitz G, Chernozemsky I, Sirakova L, Chilingirov P, Souchon R, Marschner N, et al. Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs. 2005; 16: 871-877.
- Schmittel A, Knödler M, Hortig P, Schulze K, Thiel E, Keilholz U, et al. Phase II trial of second-line bendamustine chemotherapy in relapsed small cell lung cancer patients. Lung Cancer. 2007; 55: 109-113.
- Köster W, Heider A, Niederle N, Wilke H, Stamatis G, Fischer JR, et al. Phase II trial with carboplatin and bendamustine in patients with extensive stage small-cell lung cancer. J Thorac Oncol. 2007; 2: 312-316.
- Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008; 14: 309-317.
- Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs. 1996; 7: 415-421.
- Bremer K. High rates of long-lasting remissions after 5-day bendamustine chemotherapy cycles in pre-treated low-grade non-Hodgkin's-lymphomas. J Cancer Res Clin Oncol. 2002; 128: 603-609.
- Leoni LM, Niemeyer CC, Kerfoot C. In vitro and ex vivo activity of SDX-105 (bendamustine) in drug-resistant lymphoma cells [abstract 1215]. Proc Am Assoc Cancer Res. 2004; 45: 278.
- Chou TC, Arlin Z, Clarkson BD, Phillips FS. Metabolism of 1-beta-D-arabinofuranosylcytosine in human leukemic cells. Cancer Res. 1977; 37: 3561-3570.
- Kessel D, Hall TC, Wodinsky I. Transport and phosphorylation as factors in the antitumor action of cytosine arabinoside. Science. 1967; 156: 1240-1241.
- Kufe DW, Major PP, Egan EM, Beardsley GP. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980; 255: 8997-8900.
- Spriggs D, Robbins G, Ohno Y, Kufe D. Detection of 1-beta-D-arabinofuranosylcytosine incorporation into DNA in vivo. Cancer Res. 1987; 47: 6532-6536.
- Peters WG, Colly LP, Willemze R. High-dose cytosine arabinoside: pharmacological and clinical aspects. Blut. 1988; 56: 1-11.
- Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993; 11: 116-124.
- Staib P, Schinkothe T, Dimsky T. In vitro modulation of Ara-ctp accumulation in fresh AML cells by Bendamustine in comparison with Fludarabine, 2-CdA and Gemcitabine. Blood. 1999; 94: Abst. 271.
- Chow KU, Boehrer S, Napieralski S, Nowak D, Knau A, Hoelzer D, et al. In AML cell lines Ara-C combined with purine analogues is able to exert synergistic as well as antagonistic effects on proliferation, apoptosis and disruption of mitochondrial membrane potential. Leuk Lymphoma. 2003; 44: 165-173.
- Hiraoka N, Kikuchi J, Yamauchi T, Koyama D, Wada T, Uesawa M, et al. Purine analog-like properties of bendamustine underlie rapid activation of DNA damage response and synergistic effects with pyrimidine analogues in lymphoid malignancies. PLoS One. 2014; 9: e90675.
- Visco C, Castegnaro S, Chieregato K, Bernardi M, Albiero E, Zanon C, et al. The cytotoxic effects of bendamustine in combination with cytarabine in mantle cell lymphoma cell lines. Blood Cells Mol Dis. 2012; 48: 68-75.
- Castegnaro S, Visco C, Chieregato K, Bernardi M, Albiero E, Zanon C, et al. Cytosine arabinoside potentiates the apoptotic effect of bendamustine on several B- and T-cell leukemia/lymphoma cells and cell lines. Leuk Lymphoma. 2012; 53: 2262-2268.
- Schwänen C, Hecker T, Hübinger G, Wölfle M, Rittgen W, Bergmann L, et al. In vitro evaluation of bendamustine induced apoptosis in B-chronic lymphocytic leukemia. Leukemia. 2002; 16: 2096-2105.
- Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008; 26: 4473-4479.
- Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013; 31: 2103-2109.
- Damaj G, Gressin R, Bouabdallah K, Cartron G, Choufi B, Gyan E, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013; 31: 104-110.
- Visco C, Finotto S, Zambello R, Paolini R, Menin A, Zanotti R. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013; 31: 1442-1449.
- Visco C, Finotto S, Pomponi F, Sartori R, Laveder F, Trentin L, et al. The combination of rituximab, bendamustine, and cytarabine for heavily pretreated relapsed/refractory cytogenetically high-risk patients with chronic lymphocytic leukemia. Am J Hematol. 2013; 88: 289-293.