
Case Report
Austin J Cancer Clin Res 2015;2(3): 1036.
Synchronous Double Cancer Developing in the Uterine Cervix and Endometrium of a Patient Taking Tamoxifen after Surgery for Breast Cancer
Yamauchi A1,2, Yokoyama Y2*, Morikawa A1, Soma T1, Ota K1, Yokota M1, Matsukura D1, Sato S1 and Mizunuma H2
1Department of Obstetrics and Gynecology, Aomori Prefectural Central Hospital, Japan
2Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, Japan
*Corresponding author: Yokoyama Y, Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, 5-Zaifu-cho, Hirosaki, Aomori, 036- 8562, Japan.
Received: February 10, 2015; Accepted: March 20, 2015; Published: April 03, 2015
Abstract
Aim: Tamoxifen (TAM) is known to be a risk factor for endometrial cancer, and it is also reported to be a risk factor for cervical cancer.
Case: As reported here, endometrial cancer and adenocarcinoma of the cervix developed during the second year that a patient was taking TAM after surgery for breast cancer. Endometrial cancer was a stage IA well-differentiated endometrioid adenocarcinoma, and cancer of the cervix was a stage IB1 cervical adenocarcinoma. Breast cancer has not recurred for 2 years since surgery. TAM resulted in synchronous double cancer in the form of endometrial cancer and adenocarcinoma of the cervix. Including the patient’s original breast cancer, the patient had metachronous triple cancer.
Conclusion: Lesions of the cervix and endometrium must not be overlooked while a patient is taking TAM.
Keywords: Tamoxifen; Endometrial cancer; Adenocarcinoma of the uterine cervix; Breast cancer
Introduction
There is established evidence for taking tamoxifen (TAM) as an endocrine therapy following surgery for breast cancer. Over the past few years, many patients who have undergone surgery for breast cancer have received this treatment. TAM has been reported to lead to adverse reactions such as irregular vaginal bleeding, menstrual abnormalities, endometrial polyps, and endometrial hyperplasia, but attention should mostly be focused on development of endometrial cancer. Fisher et al. reported that patients administered TAM after surgery for breast cancer had a 7.5-fold higher incidence of endometrial cancer compared to patients who were not administered TAM [1]. Thus, the American Congress of Obstetricians and Gynecologists recommends that patients taking TAM after surgery for breast cancer undergo a gynecologic examination annually [2]. In the current case, a patient taking TAM after surgery for breast cancer underwent an annual gynecologic examination at this department. Abnormal endometrial cytology was noted 2 years after the patient had taken TAM, and adenocarcinoma of the cervix was also present. These aspects make this case extremely rare. This case is discussed here from 2 perspectives. One is the effect that taking TAM after surgery for breast cancer had on development of cervical and endometrial cancer. The other is the combined incidence of endometrial cancer, adenocarcinoma of the cervix, and breast cancer.
Case Presentation
The patient was a 45-year-old woman, gravida 1, para 1 with a height of 160 cm and weight of 44 kg. The age at menarche was 13 years, and the patient had a normal 30-day menstrual cycle. The patient underwent partial right mastectomy and sentinel node biopsy for cancer of the right breast. Staging indicated that the cancer was stage I, T1N0M0. In the first month following surgery, the patient began taking tamoxifen 20 mg/d. In the second and third month following surgery, the remaining portion of the right breast was irradiated to a dose of 50 Gy, and the inner surgical margin was irradiated with a boost dose of 10 Gy. Four months after surgery, the patient began taking an LH-RH agonist as well. The patient’s family history was unremarkable. A CT scan performed for follow-up 6 months after surgery for breast cancer revealed a uterine myoma, so the patient was seen initially by this Department for further testing. The uterus was fist-sized and several small myomas were noted, but there was no endometrial thickening and both ovaries were normal size. Cervical cytology was negative for intraepithelial lesion or malignancy (NILM) and endometrial cytology was normal (class II), so an approach of following up periodically was taken. When the patient was seen a year and a half after her initial examination, she complained of irregular vaginal bleeding but there was no endometrial thickening, cervical cytology was NILM, endometrial cytology was normal (class II). Subsequently, irregular vaginal bleeding was noted about once every 2-3 months. During a routine examination a year later, endometrial thickening was not noted, but cervical cytology revealed swollen nuclei, increased chromatin, and clumps of cells that were thought to be glandular. Atypical glandular cells were identified. Endometrial cytology was abnormal (class IIIa) same as the cervical cytological findings. Two months later, an endometrial biopsy was performed and cervical cytology was performed again. Endometrial biopsy revealed an adenocarcinoma, and the results of the second cervical cytology were class V (adenocarcinoma). Thus, colposcopy was performed (Figure 1). There was a hemorrhagic “macrocarcinoma” with atypical vasculature in the cervix from the 6 o’clock-9 o’clock positions. This mass was biopsied.
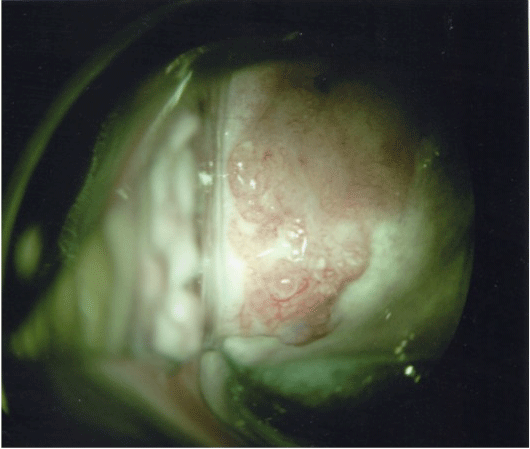
Figure 1: Colposcopy showed a macrocarcinoma with atypical vasculature in
the cervix from the 6 o, clock-9 o, clock positions.
Cervical biopsy (Figure 2a): Columnar tumor cells were growing in solid nests with an ill-defined cribriform pattern. Some of the cells had mucoid substances. The tumor was deduced to be a moderately differentiated-poorly differentiated endocervical adenocarcinoma.
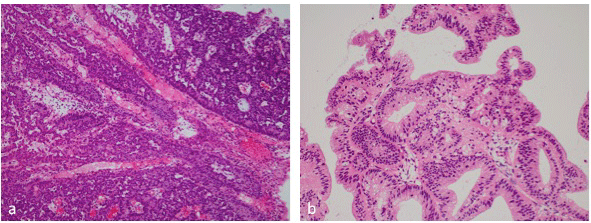
Figure 2: a, Cervical biopsy showed a moderately differentiated-poorly
differentiated endocervical adenocarcinoma. X100. b, endometrial biopsy
showed a well-differentiated endometrioid adenocarcinoma. X100.
Endometrial biopsy (Figure 2b): There were areas where papillary projections and nuclear stratification were evident in dilated ducts, and there were areas with a combined pattern featuring irregular duct formation. Almost all of the tumor cells were positive for MIB-1. The tumor was deduced to be a well-differentiated adenocarcinoma.
Tumor marker levels were SCC of 1.2 ng/ml, CA19-9 of 36.5 U/ ml, and CA125 of 14.8 U/ml. A pelvic MRI (Figure 3) revealed an illdefined lesion of the cervix, no thickening of the endometrium, and no evidence of myometrial invasion. There was no evidence of swollen axillary lymph nodes or distant metastasis in contrast-enhanced CT scans from the neck to the pelvis. Thus, the cancers were diagnosed as stage IB1 adenocarcinoma of the cervix and stage I endometrial cancer, and a radical hysterectomy and bilateral adnexectomy were performed. Resected specimens are shown in Figure 4. Lesions were not macroscopically evident in the cervix and uterine corpus.
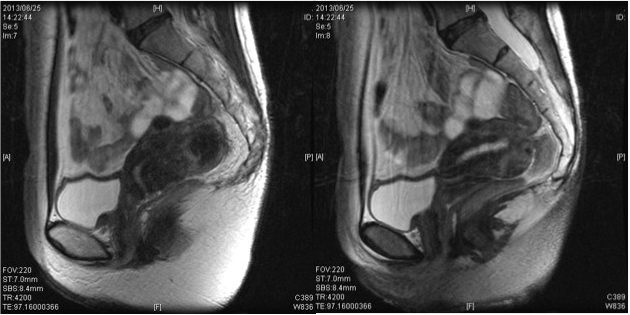
Figure 3: MRI Showed an ill-defined lesion of the cervix, no thickening of the
endometrium, and no evidence of myometrial invasion.
Tumor marker levels were SCC of 1.2 ng/ml, CA19-9 of 36.5 U/ ml, and CA125 of 14.8 U/ml. A pelvic MRI (Figure 3) revealed an illdefined lesion of the cervix, no thickening of the endometrium, and no evidence of myometrial invasion. There was no evidence of swollen axillary lymph nodes or distant metastasis in contrast-enhanced CT scans from the neck to the pelvis. Thus, the cancers were diagnosed as stage IB1 adenocarcinoma of the cervix and stage I endometrial cancer, and a radical hysterectomy and bilateral adnexectomy were performed. Resected specimens are shown in Figure 4. Lesions were not macroscopically evident in the cervix and uterine corpus.
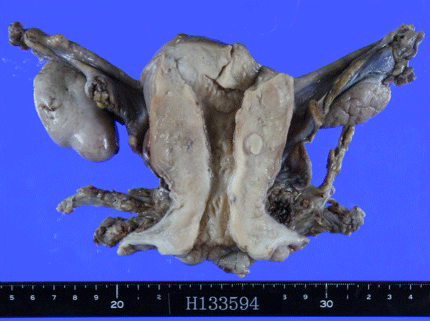
Figure 4: Resected specimens. Lesions were not macroscopically evident in
the cervix and uterine corpus.
Postoperative pathologic diagnosis: 1) The cervix: There was evidence of glandular dysplasia and adenocarcinoma in situ in the cervix, and there were areas with tumor cells in papillary formations, cells with a cribriform pattern, and solid nests of cells. Glandular involvement was evident along with stromal invasion in some areas. There was no vascular involvement. The lesion had a length of 8 mm and depth of 2.2 mm, and continuous spread to the endometrium was not noted. Tumor cells had mucus, and the tumor was diagnosed as a mucinous adenocarcinoma. In immunostaining, many portions of the tumor had cells with Alcian blue-positive mucus, and cells were strongly positive for CEA and p16. 2) The uterine corpus: Hyperplasia was present in most portions of the endometrial lesion, though there were a few areas with ducts growing in a papillary fashion in the uterine cavity, consolidated ducts, and cells in a cribriform pattern. The lesion was localized to the endometrium, and invasion of the myometrium was not noted. The tumor was diagnosed as grade 1 endometrioid adenocarcinoma associated with atypical endometrial hyperplasia. In immunostaining, cells in the lumen of tumor glands were positive for PAS, but there was no evidence of mucus in cells and cells were negative for CEA and p16. The final diagnosis was a double cancer of stage IB1 adenocarcinoma of the cervix and stage IA endometrial cancer.
Postoperative course: The patient also had dysuria, infection of a lymphocele, and urinary tract infection. With conservative treatment involving medication, these problems abated. On day 21 postoperatively, the patient was discharged. Cancer has not recurred for 2 years since surgery.
Discussion
TAM is readily known to act like estrogen on the endometrium. Yamazawa et al. examined the changes that occurred in the endometrium as a result of taking TAM [3]. The most common result is thinning of the endometrium, though other changes include proliferative changes, secretory changes, pseudodecidual changes, endometrial polyps, endometrial hyperplasia, and adenocarcinoma. Deligdish et al. examined endometrial histology in 700 patients who received TAM after surgery for breast cancer, and they reported finding some form of lesions in 40% of those patients and endometrial cancer in 7% [4]. Of 33 patients with endometrial cancer, the histologic type of that cancer was most often endometrioid adenocarcinoma (20 patients), followed by serous adenocarcinoma (8 patients) [4]. The Gynecologic Tumor Committee of the Japan Society of Obstetrics and Gynecology stated that the histologic type of endometrial cancer in individuals taking TAM was most often clear cell adenocarcinoma, though a study reported being unable to corroborate the contention that most of these cancers were endometrioid adenocarcinomas or that they had a specific type [5]. The histologic type of endometrial cancer in the current case was a well-differentiated endometrioid adenocarcinoma with no invasion of the myometrium. This cancer is presumed to have a good prognosis. Leeuwen et al. examined the relationship between the duration of TAM administration and endometrial carcinogenesis, and they reported that taking TAM for 2 years or longer increased the risk of carcinogenesis 2.3-fold while taking it for 5 years or longer increased the risk of carcinogenesis 3.0- fold [6]. Yokosuga et al. found that the risk of endometrial cancer increased while taking TAM for 2 years or longer, so they cited the need for routine screening over the long-term, even if the patient was symptom-free [7]. In the current case, endometrial cancer developed over the 2 years the patient was taking TAM. TAM is likely to be associated with endometrial carcinogenesis. In the first year of taking TAM, the patient complained of irregular bleeding, but endometrial cytology was normal, so the bleeding was considered an adverse reaction to TAM and the patient was followed. In addition, little endometrial thickening was evident and there was scant evidence of endometrial cancer. Endometrial cancer was diagnosed early via a periodic gynecologic examination. One effect of TAM on the endometrium is the transformation of endometrial polyps into cancer. Endometrial cytology may have limited ability to ascertain this development, so periodically performing endometrial biopsies is also crucial.
What effect does TAM have on cervical cancer? Hwang et al. used cervical cancer cells to examine the effects of TAM, and they reported that TAM significantly enhanced HPV16 expression and expression of HPV16-derived E7 protein [8]. Later, Kim et al. clarified the molecular biological mechanisms by which TAM regulates HPV16/18 and E6/E7 expression in cervical cancer cells, and they stated that TAM was a risk factor for cervical carcinogenesis [9]. In the cervix, TAM may be exposed to hormonal conditions similar to those in the endometrium. A study stated that TAM was significantly associated with development of adenocarcinoma of the cervix [10]. In the current case, the cervix went from normal to exhibiting invasive carcinoma in a brief period of 1 year. HPV testing was not performed in the current case, but this case strongly suggests that TAM is associated with cervical carcinogenesis. This case involved synchronous double cancer in the form of endometrial cancer and adenocarcinoma of the cervix. TAM may have been associated with the carcinogenesis of these 2 cancers.
There are several reports of double cancer in the form of cervical cancer and breast cancer. Moertel et al. defined synchronous double cancer as additional cancer developing within 6 months of the first cancer and they defined metachronous double cancer as additional cancer developing after an interval of 6 months or longer [11]. In Japan, synchronous double cancer involving cervical cancer has an incidence of 0.46-2.94%; the additional cancer is most often uterine cancer, followed by ovarian cancer, breast cancer, and then gastric cancer [12]. Metachronous double cancer involving cervical cancer has an incidence of 0.82-1.33%; the additional cancer is most often gastric cancer, followed by breast cancer, colon cancer, and then lung cancer [12]. In the cited source, the histologic type of cervical cancer was most often squamous cell carcinoma, and adenocarcinoma was rare [12]. Based on that source, synchronous double cancer in the form of cervical cancer and endometrial cancer is somewhat likely to occur, as is metachronous double cancer in the form of cervical cancer and breast cancer. In the current case, however, all 3 cancers developed in a single patient, and the histologic type of cervical cancer was adenocarcinoma. Assuming one set aside the potential association between taking TAM and cancer development, this case is extremely rare and extremely intriguing.
Conclusion
We encountered a significant case in which both adenocarcinoma of the cervix and endometrial cancer developed in a patient taking TAM after surgery for breast cancer. When patients are taking TAM after surgery for breast cancer, they must undergo periodic gynecologic examinations given the potential for development of endometrial cancer. Efforts must be made to detect such cancer early on. In addition, TAM may be a risk factor for cervical carcinogenesis. Lesions of the endometrium and cervix must not be overlooked while a patient is taking TAM.
References
- Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, CroninWM. Endometrial Cancer in Tamoxifen-Treated Breast Cancer Patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994; 86: 527-537.
- Committee on Gynecologic Practice, The American College of Obstetricians and Gynecologists. ACOG committee opinion: Tamoxifen and endometrial cancer. Int J Gynaecol Obstet. 2001; 73: 77-79.
- Yamazawa K, Miyazawa Y, Suzuki M, Wakabayashi M, Kaku H, Matsui H, et al. Tamoxifen and the risk of endometrial cancer in Japanese women with breast cancer. Surg Today. 2006; 36: 41-46.
- Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F, et al. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000; 78: 181-186.
- Nishimura N, Hachisuga T, Saito T, Kawarabayashi T. Subsequent endometrial carcinoma with adjuvant tamoxifen treatment in Japanese breast cancer patients. Int J Gynecol Cancer. 2001; 11: 272-276.
- vanLeeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrère CH, Otter R, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994; 343: 448-452.
- Yokosuka K, Teshima H, Katase K, Fujimoto I, Yamauchi K, Hasumi K, et al. [Effects of long-term administration of tamoxifen on vaginal epithelium and complications of endometrial lesions in breast cancer patients]. Nihon Sanka Fujinka Gakkai Zasshi. 1995; 47: 125-132.
- Hwang JY, Lin BY, Tang FM, Yu WC. Tamoxifen stimulates human papillomavirus type 16 gene expression and cell proliferation in a cervical cancer cell line. Cancer Res. 1992; 52: 6848-6852.
- Kim CJ, Um SJ, Kim TY, Kim EJ, Park TC, Kim SJ, et al. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells. Int J Gynecol Cancer. 2000; 10: 157-164.
- . Watrowski R, Striepecke E, Jäger C, Bauknecht T, Horst C. Papillary-serous adenocarcinoma of the uterine cervix during tamoxifen therapy after bilateral breast cancer. Anticancer Res. 2012; 32: 5075-5078.
- Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961; 14: 221-230.
- Takatori E, Shoji T, Miura Y, Takeuchi S, Uesugi N, Sugiyama T. Triple simultaneous primary invasive gynecological malignancies: a case report. J Obstet Gynaecol Res. 2014; 40: 627-631.