
Review Article
J Cardiovasc Disord. 2015; 2(2): 1013.
Arrhythmic Risk Stratification in Adults with Congenital Heart Disease
Daliento L*, Mazzotti E, Pomiato E, Spadotto V and Bauce B
Department of Cardiac, University of Padua, Italy
*Corresponding author: Daliento L, Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, 2 via Giustiniani, 35100, Padua, Italy
Received: June 23, 2015; Accepted: July 27, 2015; Published: July 29, 2015
Abstract
As arrhythmias and sudden death may significantly modify natural history of Congenital Heart Disease (CHD), accurate arrhythmic risk stratification is mandatory during clinical follow-up of both native and operated CHD patients, especially in adulthood. This can be obtained using simple and reproducible instruments such as Electrocardiogram (ECG), 24 -hours Holter ECG, signalaveraged ECG and echocardiography. Nonetheless, cardiac magnetic resonance and electrophysiological study with programmed ventricular stimulation and electro-anatomic mapping may help to define a more effective patient management. Correction of residual hemodynamic lesions significantly improves arrhythmia-free survival. Some classes of patients with higher arrhythmic risk have been already identified and, when all residual lesions have been addressed, ICD implantation in primary prevention may represent a valid option. On the other hand, pharmacological therapy of arrhythmias is often non- CHD specific.
Keywords: Congenital heart disease; Grown up heart disease; Arrhythmias; Sudden death
Introduction
Arrhythmias, together with ventricular dysfunction, are the most important prognostic determinants of natural history of Congenital Heart Disease (CHD), especially in adults after surgical correction, representing the main cause of death and hospitalisation [1,2].
Arrhythmias observed in CHD population
Regarding bradyarrhythmias, perioperative complete Atrioventricular (AV) block is overall rare in the current surgical era, while late complete AV block is observed in patients with history of perioperative transient AV block, with right bundle branch block and left anterior fascicular block, with sick sinus syndrome or with corrected transposition of great arteries (especially when associated with ventricular septal defect) [3]. Sinus node dysfunction can be produced by injury of sinus node or its vessel during cardiopulmonary by-pass (performing cannulation at the junction between the superior vena cava and the roof of the right atrium) or during severe surgical atrial manipulations, such as in Mustard/Senning or atrio-pulmonary operations [4].
On the other hand, hyperkinetic arrhythmias are most commonly observed in both native and surgically corrected CHD, and may result in clinical worsening and sudden death.
In a population-based study Silka et al. [5] reported the postsurgical follow-up of 3589 adult (>19 years old) patients who underwent surgical treatment of common forms of congenital heart disease (atrial septal defects, ventricular septal defects, atrioventricular septal defects, patent ductus arteriosus, pulmonary stenosis, congenital aortic valve stenosis, aortic coarctation, tetralogy of Fallot, complete transposition of the great vessels) in the state of Oregon between 1958 and 1996, retrospectively identifying incidence and causes of late sudden death, with a mean follow-up of 25.3 years (median 25.4, range 1.06 to 35.5). They observed 41 unexpected late sudden deaths during 45,857 patient-years of follow-up (overall event rate 0.9‰ pt/yr, 25–100), 37of which occurred in patients with transposition of great arteries, tetralogy of Fallot, aortic valvular stenosis or aortic recoarctation (event rate 2.2‰ pt/yr), while only 4 were recorded in patients with ventricular septal defect, atrioventricular septal defect or pulmonary stenosis (event rate 0.1‰ pt/yr). Actuarial 10-, 20-, 30- and 36-year survival rates in the 490 patients who survived the first postoperative year were 97%, 94%, 89% and 85%, respectively.
Long term survival after surgical correction of tetralogy of Fallot is about 85% after 35 years of follow-up, but sudden death and ventricular tachycardia occurred with a reported incidence of 1–2% over 5 years for young adults, and an overall prevalence of sudden cardiac death of 3–6% has been reported [6]. In 2000, Gatzoulis et al. [7] analyzed retrospective data from a 10-year period (1985-95) on the occurrence of clinical arrhythmia and sudden death among 793 patients with repaired Tetralogy of Fallot (excluding patients with pulmonary atresia and patients with additional major intra cardiac or extra cardiac abnormalities) who had been referred to six different congenital heart disease centres, allowing for over 7500 patient-years follow-up. Patients mean age was 17.5±10.7 years, mean age at repair was 8,4±6,1 years. Among them, 33 patients developed sustained monomorphic ventricular tachycardia, and 16 died suddenly. The authors reported significantly higher QRS duration and QRS rate of change in 10-years period among the ventricular tachycardia and sudden-death groups, and pulmonary regurgitation as the main underlying hemodynamic lesion within the same group; moreover, they did find an association between older age at repair and risk of sudden death.
Besides the role of life-threatening arrhythmias in causing sudden death, long-standing supraventricular or non-sustained ventricular arrhythmias may cause or be caused by a decrease in ventricular function, thus influencing patient prognosis, such as for Mustard patients, who were reported to have a reduced survival in the presence of both systemic ventricular dysfunction and atrial fibrillation [8].
Supraventricular tachyarrhythmia’s have different clinical and prognostic value, depending on the type of CHD and surgical history. Atrial fibrillation was reported to increase the risk of heart failure in a group of 521 patients > 40 years old with secundum atrial septal defect referred for treatment and who received a median follow-up of 7.3 (range 2-13) years [9]. In Roos-Hesselink et al. work, after 17.5 years (range1.4 to 32 years) of post-surgical follow up of 53 consecutive patients (mean age 26.6 years) with repaired Tetralogy of Fallot (mean age at repair 9.1 years, range, 0.7-55 years), about 20% of patients of suffered from atrial fibrillation, having systemic embolic events in 7% [10]. Atrial flutter in patients who underwent Mustard operation has already been related to sudden death: in a retrospective, multicenter, case-controlled study, 47 patients after Mustard’s or Senning’s operation who experienced a sudden-death event were found to have an history of documented arrhyhtmia, mainly atrial flutter (OR 3.473, CI 95 1.451-8.310) [11]. About 16% of the 145 patients analysed by Kirsh et al. after Mustard/Senning operation, Fontan operation, biventricular repair of complex CHD or other palliative intervention and enrolled after a first atrial cardio version attempt [12] had a recurrence of supraventricular tachyarrhythmia’s (atrial flutter/fibrillation, or intra-atrial arrhythmic re-entry tachycardia — IAART) in a 10-years follow up, with a higher risk of thromboembolism, heart failure and death. One hundred ninetynine patients who underwent the Fontan operation between 1975 and 1993 were followed for more than 10 years in Fujita et al. work [13], and authors reported a probability of freedom from atrial tachycardia of 86% and 62% at 10 and 20 years of follow up. Type of surgery (especially atrio-pulmonary Fontan), duration of postsurgical follow-up and age at operation have been reported to best predict late arrhythmic risk in Fontan populations. In Fishberger et al. [14] group of 334 patients who underwent the Fontan operation between 1973 and 1991, after a mean follow-up of 5.0 ± 3.8 years, late occurrence of arrhythmias was reported to be higher in patients with older age at surgery (12.4 ± 7.6 vs 6.3 ± 5.2 years, p < 0.001) and after a longer follow-up interval (8.7 ± 3.9 vs 4.4 ± 3.4 years; p < 0.001). More recently, a Paediatric Heart Network investigation reported a significant reduction of IAART risk in a contemporary Fontan cohort until 4–6 years of follow up, increasing with patient age [15].
With all this in mind, an accurate identification of CHD patients with high arrhythmic risk is mandatory during long-term follow-up.
This can be obtained using simple and reproducible instruments such as Electrocardiogram (ECG), 24 -hours Holter ECG, signalaveraged ECG and echocardiography. Nonetheless, cardiac magnetic resonance and electrophysiological study with programmed ventricular stimulation and electro-anatomic mapping may help to define a more effective patient management. Medical history collection should include data about native congenital cardiac defect, type of cardiac surgery, age at operation, functional status, and presence of syncope.
Identification of arrhythmic substrates in CHD patients
Arrhythmias in CHD patients may have several causes: anatomical (fibrosis, surgical scar) (Figure 1) [16,17], functional (autonomic nervous system abnormalities) and morpho-functional (such as mechano-electrical interaction).
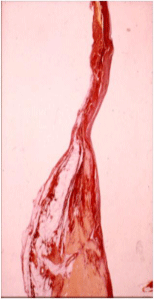
Figure 1: Fibro-fatty myocardial replacement around right ventricular
infundibular patch in a young girl with repaired Tetralogy of Fallot, who
experienced late sudden death.
An increased amount of fibrosis may be related to a long-standing systemic desaturation, to adverse ventriculo-ventricular interaction or to the presence of intrinsical myocardial anomalies. All of them may potentially affect ventricular depolarisation and repolarisation processes, a fact that can be accurately identified by SAECG (either with time or frequency domain analysis). SAECG role in identifying patients with repaired tetralogy of Fallot at risk of ventricular tachycardia and sudden cardiac death has already been demonstrated (Table 1) [18,19].
Isolated EVB (n=22)
VT/VF (n=9)
P
Mean repair age (years)
8.25 ± 6
13.56 ± 9
<0.05
Unfiltered QRS (ms)
140.93 ± 30.7
159.6 ± 31.2
NS
QT dispersion (ms)
62.18 ± 29.2
115.66 ± 58.5
<0.01
QTc dispersion (ms)
0.68 ± 0.3
1.2 ± 0.6
<0.01
RVEDV (ml/m2)
106.9 ± 27.6
160.33 ± 58
<0.01
LVEDV(ml/m2)
57.37 ± 13
93.66 ± 50.5
<0.01
LVEF (%)
62.90 ± 8.05
49.66 ± 10.2
<0.01
Values are indicated as mean±SD.
Table 1: Fibro-fatty myocardial replacement around right ventricular infundibular patch in a young girl with repaired Tetralogy of Fallot, who experienced late sudden death.
A mechano-electrical interaction has been shown in patients with CHD, particularly in those with repaired tetralogy of Fallot, in whom chronic volume overload caused by pulmonary valvular regurgitation increases electrical instability [7,20]. The presence of a prolonged non-filtered QRS duration associated with an increase of right ventricular end diastolic volume at follow-up seems to better predict arrhythmic risk than the absolute QRS duration, as they dynamically reflect an increase of fibrosis amount, together with a progressive hemodynamic impairment [21] (Figure 2).
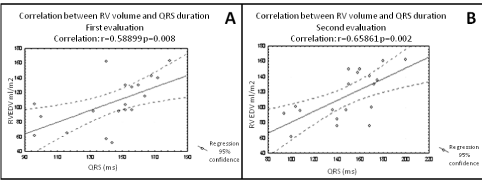
Figure 2: Baseline (A) and at 5-years-follow up (5) correlation between QRS
duration and RV volume in patients with repaired TOF. Correlation between
two variables was evaluated by means of linear regression analysis and
expressed as the correlation coefficient (r). A dilatation of the right ventricle
(RVEDV from 110 ml/mq to 150 ml/mq) associated with a significant increase
in QRS duration (from 145 to 185 ms) is observed. RV= Right Ventricle;
RVEDV= Right Ventricular End Diastolic Volume. Adapted from [21].
Spatial and temporal dispersion of QT interval has been demonstrated to reflect inhomogeneous ventricular repolarisation: values higher than 80 ms for spatial dispersion and 65 ms for temporal dispersion identified, in a population of 66 patients with previous surgical repair of Tetralogy of Fallot, those at higher risk of developing ventricular tachycardia (Table 1) [22]. Increased QT dispersion and loss of sinus rhythm have also been associated with an higher risk of sudden cardiac death in a group of 312 patients who had undergone atrial switch operation for a complete transposition of the great vessels, of whom 22 (7%) experienced sudden cardiac death [23].
Physical effort may concur to induce sudden death, suggesting a role of the adrenergic nervous system; MIGB-SPECT analysis already evidenced qualitative and quantitative abnormalities of both presence and distribution of adrenergic fibres in the ventricular myocardium of patients with repaired Tetralogy of Fallot [24] (Figure 3). Heart Rate Variability (HRV) assessment is a simple and reproducible instrument to highlight autonomous nervous system anomalies (Table 2A), and modifications of HRV at follow-up seems to predict development of repetitive ventricular arrhythmias (Table 2B).
A
Control group (n=35)
Study group (n=38)
p
Heart rate (ms)
820±112
812±107
0.756
SDNN (ms)
156.1±32
124.6±45
0.001
RMSSD (ms)
37.7±16
47.8±34
0.114
pNN50 (%)
11.7±9
13.5±15
0.540
SDANN (ms)
139.9±30
113.2±43
0.003
Values are indicated as mean±SD.
B
No VT (n=30)
VT (n=8)
p
Heart rate (ms)
814±111
801±95
0.754
SDNN (ms)
133.4±46
91.7±19
0.017
RMSSD (ms)
52.5±36
30.3±14
0.101
pNN50 (%)
15.9±16
4.6±3
0.055
SDANN (ms)
119.9±46
88.1±22
0.065
Values are indicated as mean±SD.
Table 2: Heart rate variability in a group of 38 consecutive adult patients (mean age 31±10 years) with repaired Tetralogy of Fallot (excluding those with severe pulmonary regurgitation, previous episodes of sustained or non–sustained ventricular tachycardia or ventricular fibrillation, chronic treatment with anti arrhythmic drugs, indications for cardiac reoperation). A) Time domain indexes of heart rate variability in controls and in patients (“study group”). B) Time domain indexes of heart rate variability in the study group, according to the presence of sustained ventricular tachycardia at follow-up. Differences between groups were examined for statistical significance by analysis of variance. Statistical significance was assumed with a level of P<0.05. SDNN=Standard Deviation of all NN intervals; RMSSD=square root of the mean square differences of successive NN intervals; pNN50= percentages of the number of interval differences of successive NN intervals > 50 m; SDANN= Standard Deviation of the Average NN intervals calculated over 5-minute intervals; VT= Ventricular Tachycardia. Adapted from [53].
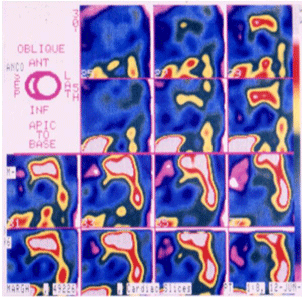
Figure 3: MIGB scintigraphy showing the presence of structural abnormalities
involving nerve endings and receptors in patients after tetra logy of Fallot
repair. In homogeneity of adrenergic fibres is more evident in patients with
higher right ventricular end-diastolic volumes and lower ejection fraction, and
associated with severe ventricular arrhythmias [18,24].
Ventricular dysfunction [25,26] is often associated with electrical instability, particularly during physical effort [7, 21]. An echocardiographic evaluation is then mandatory for a complete arrhythmic risk evaluation, and right and left ventricular function assessment have already been demonstrated to play a role in identification of patients with repaired Tetralogy of Fallot at higher risk of ventricular tachycardia/sudden cardiac death (Table 3), or patients with increased risk of developing atrial fibrillation after the Mustard operation [8].
CONTROLS
ALL
MINOR ARRHYTHMIAS
SEVERE ARRHYTHMIAS
Filtered QRS duration (ms)
125 ± 4 *
162 ± 29
156 ± 29 #
181.5 ± 19.6
LAS 40 (ms)
33.6 ± 13.4
32 ± 22
28.5 ± 19.8 §
45.1 ± 26.7
RMS 40 (mV)
26 ± 8
41 ± 32
45.3 ± 34.6
26 ± 16
Values are expressed as mean ±SD.
*p<0.001 vs patients with minor and severe arrhythmias
# p< 0.01 vs patients with severe arrhythmias
§ p<0.03 vs patients with severe arrhythmias
Table 3: Arrhythmic risk stratification with Signal-Averaged (SA) ECG in sixty-six consecutive patients, mean age 26 ± 10 years, studied 17.7 ± 5.8 years after total correction for tetralogy of Fallot, according to SAECG parameters. During a mean follow-up period of 7.3 ± 3.1 years, 12 patients had episodes of sustained Ventricular Tachycardia (VT) and two of them suddenly died. All patients had complete right bundle branch block. Analysis of variance and Scheffe’s test were used to compare means among the different groups. Dunnett’s test was used to compare means for the two groups with arrhythmias with those of the control group. Patients with VT were characterized by a significantly longer filtered QRS duration (fQRS) at all filter settings. On the contrary, there was no difference in standard QRS duration in patients with or without VT. At multivariate analysis, left ventricular ejection fraction and fQRS were independent predictors for VT. LAS 40 =duration of high-frequency low-amplitude signal <40 ms; RMS 40 = Root Mean Square of the mean voltage in the terminal portion (last 40 ms) of the filtered QRS. Adapted from [19].
Speckle tracking echo allows a direct analysis of deformation of different myocardial areas by recording longitudinal, circumferential and radial strain of both ventricles (Figure 4) [27]. Moreover, strain imaging can show right ventricular dyssynchrony [28] (Figure 5).
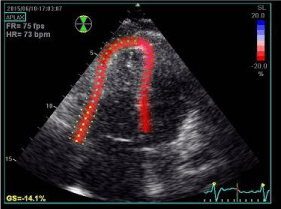
Figure 4: Speckle-tracking echocardiography (transthoracic four-chamber
apical view) showing two-dimensional right ventricle strain analysis in a
patient with repaired Tetralogy of Fallot. Global longitudinal strain is reduced
(14.1%).
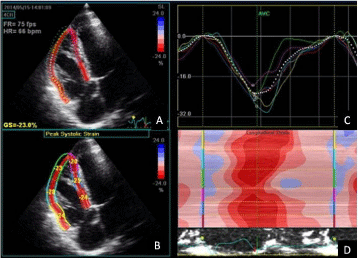
Figure 5: Speckle-tracking echocardiography (transthoracic four-chamber
apical view) showing two-dimensional right ventricle strain analysis in a
patient with repaired Tetralogy of Fallot. (A) Tracked apical loop of the
myocardial segments, GS, global strain. (B) Average segmental strain
graphically displayed. (C) Colour display of peak systolic strain. (D) M-mode
representation of peak systolic strain. Global strain is preserved but right
ventricular dyssynchrony is present (C and D). This patient experienced sub
stained ventricular tachycardia.
Cardiac Magnetic Resonance (CMR) imaging, besides a complete morpho-functional evaluation, allows, thanks to late gadolinium enhancement sequences [29] to detect fibrosis in an accurate way and with an excellent spatial resolution, as fibrotic tissue shows an high signal intensity (white areas) compared with healthy myocardium (dark areas). In patients with repaired Tetralogy of Fallot, fibrotic tissue could be detect in surgical sites, such as around the edges of infundibular or intraventricular patches [30,31], but also further down right ventricular wall; fibrosis seems to be more evident in patients with history of ventricular arrhythmias, and there seems to be a concordance between the presence and location of right ventricular low voltage areas identified by electro-anatomical voltage mapping (which were located especially around the infundibular patch and in the right ventricular anterior wall) and the presence of increased late enhancement at CMR imaging [32-34] (Figure 6). In this population, corrective surgery may damage the infundibulum (patch enlargement of the infundibulum) and include ventriculotomy, with consequent myocardial atrophy, myocyte loss and fibro-fatty replacement. The co-existence of scar and viable myocardium create opportunities for electrical re-entry, which appears to play a major role in the mechanism of sustained ventricular tachycardia in patient with repaired Tetralogy of Fallot.
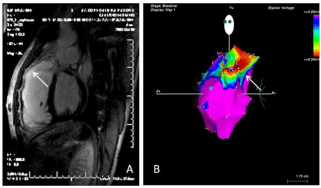
Figure 6: Detecting extent and location of fibrosis in patients with repaired
tetralogy of Fallot. A) Cardiac magnetic resonance imaging with late
gadolinium enhancement. Fibrotic regions have increased signal intensity
(brighter areas) (white left arrow). B) Three-dimensional electroanatomic
voltage mapping (CARTO system) of the right ventricle, showing low voltages
particularly in the outflow tract (right white arrow). Both findings correspond
to the presence of a scar, representing a potential substrate for ventricular
tachycardia.
With regard to invasive arrhythmic stratification tools such as electrophysiological study, event-free survival rate after 15 years of follow-up in patients with repaired Tetralogy of Fallot has been reported to be 89% for patients with negative ventricular stimulation vs. 59% for patients with inducible ventricular tachycardia. The accuracy of programmed ventricular stimulation for identification of high risk-patients was confirmed by Khairy et al. [35], with a relative risk of 5.5 for monomorphic and 12.9 for polymorphic ventricular tachycardia.
Arrhythmias management in CHD patients
Cardiac Resynchronisation Therapy (CRT) may improve clinical outcome of CHD patients with heart failure and at risk of major ventricular arrhythmias. Kutiyfa et al. reported that CRT, by improving left ventricular dyssynchrony, was associated with a significant reduction of ventricular arrhythmias in patients with left bundle branch block [36].
Appropriate selection of candidates for Intracardiac Defibrillator (ICD) implantation in primary prevention might be difficult, due to lack of major prospective studies, with a relatively low event-rate, and sometimes to anatomic challenges. Khairy et al. [37], after reviewing ICD intervention data in a population of 121 patients with repaired Tetralogy of Fallot, identified three score-based (Table 4) risk categories: low-risk 0–2 ,intermediate 3–5,high 6–12, with annualised rate of appropriate shock of 0%, 3.8% and 17.5% respectively; freedom at 10 years from appropriate ICD shocks was 100% for patients at low risk, 85 % for patients at intermediate risk and 40% for those at high risk.
Exp (β)
Points attributed
Prior palliative shunt
3.2
2
Inducible sustained VT
2.6
2
QRS = 180 ms
1.4
1
Ventriculotomy incision
3.4
2
Non-sustained VT
3.7
2
LVEDP = 12 mmHg
4.9
3
TOTAL POINTS
0-12
Table 4: Predictors of appropriate ICD shock in primary prevention in patients with repaired Tetralogy of Fallot and related risk score. Exponential values of β-coefficients and points attributed to each risk factor proposed by the authors are summarized. A 1-point increase in risk score was associated with a hazard ratio of 1.5 (95% CI 1.2 to 1.8, P<0.0003). From a possible maximum of 12 points, 3 risk categories were identified: low (0 to 2 points), intermediate (3 to 5 points), and high (>6 points). VT= Ventricular Tachycardia; LVEDP= Left Ventricular End-Diastolic Pressure. Adapted from Khairy [37].
The main therapeutic approach in CHD patients suffering from tachyarrhythmia’s should include the correction of residual hemodynamic abnormalities or surgical sequelae (such as residual outflow obstruction, significant atrioventricular valve regurgitation, residual intracardiac shunts, severe pulmonary valve incompetence in patients with repaired Tetralogy of Fallot, dilatation of the right atrium after atriopulmonary Fontan operation); reoperation seems to be more effective when associated with Maze procedure or radiofrequency ablation [38].
Catheter ablation of IAART in CHD patients has currently an early success rate of about 70% [39] and represents a reasonable approach for patients without any residual hemodynamic defect that may indicate a surgical correction. Less than 2 years after catheter ablation, however, only 40% of patients does not experience any IAART recurrence; patients with higher right atrial saturation and fewer IAART circuits, and procedural use of electro anatomic mapping are associated with a better outcome [40].
The results of chronic pharmacological therapy in preventing IAART or AF are poor, thus the need to control rhythm by nonpharmacological options, especially in patients with complex CHD. A rate-control strategy is necessary when all efforts to restore sinus rhythm have failed [41,42].
In the absence of evidence-based recommendations for antiarrhythmic drugs specific for CHD patients, most centres rely on their empirical practice, considering general principles such as presence of systemic ventricular dysfunction, abnormalities of sinus node and Atrioventricular conduction, and potential drug pro-arrhythmic effect. Acute termination of atrial or ventricular tachyarrhythmia’s in patients with CHD should be achieved by synchronized direct-current shock (Class I, level C, EHRA and AEPC-Arrhythmia Working Group joint consensus statement) [43]. Pharmacological conversion of IAART or atrial fibrillation in adults with CHD carries a risk of pro-arrhythmia [44], such as of torsades de pointes with Class III drugs, of ventricular tachycardia with Class IA and 1C drugs, and of severe sinus bradycardia or/ and systemic hypotension in adults with CHD predisposed to sinus node dysfunction, particularly with sotalol [45,46]. Amiodarone is considered one the most effective antiarrhythmic drugs, but its longterm use is limited by side effects, particularly thyroid dysfunction in women with CHD and cyanotic heart disease or univentricular hearts with Fontan palliation [47] and in those with a body mass index < 21 Kg/m2; however, it represents the drug of choice in heart failure setting. Dronedarone is a non-iodated derivative of amiodarone, with lower antiarrhythmic efficacy, but without side effects on thyroid, lungs, liver and skin and with reduced late recurrence of atrial fibrillation and the rate of hospitalization and death demonstrated by many trials (Euriodis, Adonis, Athena); however CHD population was not tested [48,49]. The new class III antiarrhythmic agents seem to reduce multi systemic side-effects, without worsening ventricular function, in particular dofetilide [50,51] showed higher rates of cardio version to sinus rhythm with longer maintenance and minor recurrence of tachyarrhythmias. The major adverse event of dofetilide is torsade de pointes, therefore its dosage has to be related to renal function, and it is contraindicated in patients with a value of QTc higher than 500ms. Dofetilide improved the success of catheter ablation in a series of CHD patients with refractory atrial tachyarrhythmias.
The PACES/HRS Expert Consensus Statement on Arrhythmias in Adult Congenital Heart Disease liberalized the use of beta-blockers in patients operated on for atrial switch to prevent ventricular arrhythmias and sudden death (Class II b, level C) and with the same class of recommendation and level of evidence suggested the use of sotalol as first choice to maintain sinus rhythm in patients with CHD, “subject to standard precautions (e.g. renal insufficiency, hypokaliemia, severe sinus node dysfunction or atrioventricular nodal disease, uncorrected QT interval >460 ms or 500 ms in the presence of ventricular conduction delay)” [52].
Conclusion
Arrhythmias, together with ventricular dysfunction, significantly influence natural history of Congenital Heart Disease (CHD), and accurate arrhythmic risk stratification should be offered to all CHD patients, especially during adulthood. Simple and reproducible instruments such as Electrocardiogram (ECG), 24 -hours Holter ECG, signal-averaged ECG and echocardiography may be effective and are readily available. Nonetheless, cardiac magnetic resonance and electrophysiological study with programmed ventricular stimulation and electro-anatomic mapping may help to further define arrhythmic risk. Correction of residual hemodynamic lesions significantly improves arrhythmia-free survival. Candidates to primary ICD implantation would need a better identification strategy with stronger studies, even if some classes of high-risk patients have been already identified. Pharmacological therapy is often non-CHD specifically validated, and further studies in this sense would then be needed.
References
- Pillutla P, Shetty KD, Foster E. Mortality associated with adult congenital heart disease: Trends in the US population from 1979 to 2005. Am Heart J. 2009; 158: 874-879.
- Kaemmerer H, Fratz S, Bauer U, Oechslin E, Brodherr-Heberlein S, Zrenner B, et al. Emergency hospital admissions and three-year survival of adults with and without cardiovascular surgery for congenital cardiac disease. J Thorac Cardiovasc Surg. 2003; 126: 1048-1052.
- Daliento L, Corrado D, Buja G, John N, Nava A, Thiene G. Rhythm and conduction disturbances in isolated, congenitally corrected transposition of the great arteries. Am J Cardiol. 1986; 58: 314-318.
- Escudero C, Khairy P, Sanatani S. Electrophysiologic considerations in congenital heart disease and their relationship to heart failure. Can J Cardiol. 2013; 29: 821-829.
- Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998; 32: 245-251.
- Nollert G, Fischlein T, Bouterwek S, Böhmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997; 30: 1374-1383.
- Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000; 356: 975-981.
- Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997; 29: 194-201.
- Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001; 38: 2035-2042.
- Roos-Hesselink J, Perlroth MG, McGhie J, Spitaels S. Atrial arrhythmias in adults after repair of tetralogy of Fallot. Correlations with clinical, exercise, and echocardiographic findings. Circulation. 1995; 91: 2214-2219.
- Kammeraad JA, van Deurzen CH, Sreeram N, Bink-Boelkens MT, Ottenkamp J, Helbing WA, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004; 44: 1095-1102.
- Kirsh JA, Walsh EP, Triedman JK. Prevalence of and risk factors for atrial fibrillation and intra-atrial reentrant tachycardia among patients with congenital heart disease. The American journal of cardiology. 2002; 90: 338-340.
- Fujita S, Takahashi K, Takeuchi D, Manaka T, Shoda M, Hagiwara N, et al. Management of late atrial tachyarrhythmia long after Fontan operation. J Cardiol. 2009; 53: 410-416.
- Fishberger SB, Wernovsky G, Gentles TL, Gauvreau K, Burnett J, Mayer JE, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997; 113: 80-86.
- Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, et al. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010; 56: 890-896.
- Becker AE, Connor M, Anderson RH. Tetralogy of Fallot: a morphometric and geometric study. Am J Cardiol. 1975; 35: 402-412.
- Schwartz SM, Gordon D, Mosca RS, Bove EL, Heidelberger KP, Kulik TJ. Collagen content in normal, pressure, and pressure-volume overloaded developing human hearts. Am J Cardiol. 1996; 77: 734-738.
- Folino AF, Daliento L. Arrhythmias after tetralogy of fallot repair. Indian Pacing Electrophysiol J. 2005; 5: 312-324.
- Russo G, Folino AF, Mazzotti E, Rebellato L, Daliento L. Comparison between QRS duration at standard ECG and signal-averaging ECG for arrhythmic risk stratification after surgical repair of tetralogy of fallot. J Cardiovasc Electrophysiol. 2005; 16: 288-292.
- Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995; 92: 231-237.
- Daliento L, Caneve F, Turrini P, Buja G, Nava A, Milanesi O, et al. Clinical significance of high-frequency, low-amplitude electrocardiographic signals and QT dispersion in patients operated on for tetralogy of Fallot. The American journal of cardiology. 1995; 76: 408-411.
- Daliento L, Rizzoli G, Menti L, Baratella MC, Turrini P, Nava A, et al. Accuracy of electrocardiographic and echocardiographic indices in predicting life threatening ventricular arrhythmias in patients operated for tetralogy of Fallot. Heart. 1999; 81: 650-655.
- Sun ZH, Happonen JM, Bennhagen R, Sairanen H, Pesonen E, Toivonen L, et al. Increased QT dispersion and loss of sinus rhythm as risk factors for late sudden death after Mustard or Senning procedures for transposition of the great arteries. Am J Cardiol. 2004; 94: 138-141.
- Daliento L, Folino AF, Menti L, Zanco P, Baratella MC, Dalla Volta S. Adrenergic nervous activity in patients after surgical correction of tetralogy of Fallot. J Am Coll Cardiol. 2001; 38: 2043-2047.
- Dietl CA, Cazzaniga ME, Dubner SJ, Pérez-Baliño NA, Torres AR, Favaloro RG. Life-threatening arrhythmias and RV dysfunction after surgical repair of tetralogy of Fallot. Comparison between transventricular and transatrial approaches. Circulation. 1994; 90: 7-12.
- Priromprintr B, Rhodes J, Silka MJ, Batra AS. Prevalence of arrhythmias during exercise stress testing in patients with congenital heart disease and severe right ventricular conduit dysfunction. The American journal of cardiology. 2014; 114: 468-472.
- Diller GP, Kempny A, Liodakis E, Alonso-Gonzalez R, Inuzuka R, Uebing A, et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation. 2012; 125: 2440-2446.
- Friedberg MK, Mertens L. Echocardiographic assessment of ventricular synchrony in congenital and acquired heart disease in children. Echocardiography. 2013; 30: 460-471.
- van der Hulst AE, Roest AA, Westenberg JJ, Kroft LJ, de Roos A. Cardiac MRI in postoperative congenital heart disease patients. J Magn Reson Imaging. 2012; 36: 511-528.
- Preim U, Sommer P, Hoffmann J, Kehrmann J, Lehmkuhl L, Daehnert I, et al. Delayed enhancement imaging in a contemporary patient cohort following correction of tetralogy of Fallot. Cardiology in the young. 2014: 1-8.
- Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999; 100: 1992-2002.
- Tsuchiya T. Three-dimensional mapping of cardiac arrhythmias - string of pearls - Circ J. 2012; 76: 572-581.
- Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, et al. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012; 5: 91-100.
- Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006; 113: 405-413.
- Khairy P, Landzberg MJ, Gatzoulis MA, Lucron H, Lambert J, Marçon F, et al. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation. 2004; 109: 1994-2000.
- Kutyifa V, Pouleur AC, Knappe D, Al-Ahmad A, Gibinski M, Wang PJ, et al. Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc Imaging. 2013; 6: 432-444.
- Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008; 117: 363-370.
- Giamberti A, Chessa M, Abella R, Butera G, Negura D, Foresti S, et al. Surgical treatment of arrhythmias in adults with congenital heart defects. Int J Cardiol. 2008; 129: 37-41.
- Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010; 56: 1589-1596.
- Triedman JK. Arrhythmias in adults with congenital heart disease. Heart. 2002; 87: 383-389.
- Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Can J Cardiol. 2014; 30: 1-63.
- Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M, et al. Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: a post-hoc analysis of the RACE II study. Eur J Heart Fail. 2013; 15: 1311-1318.
- Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013; 15: 1337-1382.
- Fish FA, Gillette PC, Benson DW. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991; 18: 356-365.
- Pfammatter JP, Paul T, Lehmann C, Kallfelz HC. Efficacy and proarrhythmia of oral sotalol in pediatric patients. J Am Coll Cardiol. 1995; 26: 1002-1007.
- Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011; 13: 329-345.
- Thorne SA, Barnes I, Cullinan P, Somerville J. Amiodarone-associated thyroid dysfunction: risk factors in adults with congenital heart disease. Circulation. 1999; 100: 149-154.
- Page RL, Connolly SJ, Crijns HJ, van Eickels M, Gaudin C, Torp-Pedersen C, et al. ATHENA Investigators. Rhythm- and rate-controlling effects of dronedarone in patients with atrial fibrillation (from the ATHENA trial). Am J Cardiol. 2011; 107: 1019-1022.
- Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011; 365: 2268-2276.
- Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999; 341: 857-865.
- Wells R, Khairy P, Harris L, Anderson CC, Balaji S. Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study. Pacing Clin Electrophysiol. 2009; 32: 1313-1318.
- Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014; 11: 102-65.
- Folino AF, Russo G, Bauce B, Mazzotti E, Daliento L. Autonomic profile and arrhythmic risk stratification after surgical repair of tetralogy of Fallot. Am Heart J. 2004; 148: 985-989.