
Research Article
Austin Chem Eng. 2016; 3(1): 1024.
Preparation and Characterization of a Sulfonated Carbonbased Solid Acid Microspheric Material (SCSAM) and its use for the Esterification of Oleic Acid with Methanol
Honglei Zhang1,2, Xiang Luo1,2, Xinwei Li1,2, George Z Chen2,3, Feng He4* and Tao Wu1*
1Key Laboratory of Clean Energy Conversion Technologies, The University of Nottingham Ningbo China, P.R. China
2Department of Chemical and Environmental Engineering, Energy and Sustainability Research Division, The University of Nottingham, UK
3International Academy of Marine Economy and Technology, The University of Nottingham Ningbo China, P.R. China
4College of Biological and Environmental Engineering, Zhejiang University of Technology, China
*Corresponding author: Tao Wu, Key Laboratory of Clean Energy Conversion Technologies, The University of Nottingham Ningbo China, 199 Taikang East Road Ningbo, 315100, P.R. China
Feng He, College of Biological and Environmental Engineering, Zhejiang University of Technology, 18 ChaoWang Road, Hangzhou, Zhejiang 310032, P. R. China
Received: February 02, 2016; Accepted: February 19, 2016; Published: February 22, 2016
Abstract
In this study, a sulfonated group (-SO3H) rich carbon-based solid acid microspheric material was prepared by hydrothermal method followed by sulfonation using glucose as the raw material. Such a green, non-corrosive, and renewable carbon material was used as a heterogeneous catalyst for the esterification of oleic acid with methanol for the production of biodiesel. The carbon microspheres were characterized systematically. It was found that the carbon microspheres prepared under the optimal reaction conditions exhibited smooth surfaces, uniform particle sizes and good dispersion. The sulfonated carbon-based solid acid microspheric materials showed high acidity and good catalytic activities for the esterification of oleic acid with methanol. The influence of reaction operating conditions on the performance of esterification was studied. The optimal esterification reaction conditions were found to be: methanol/oleic acid molar ratio 12:1, catalyst loading 0.25 g (0.05 mmol H+), reaction temperature 65 °C, reaction time 8 h and mechanical stirring rate 360 rpm. It was found that the catalyst demonstrated very good reusability although there was noticeable loss in acidity due to the leaching of active sites.
Keywords: Biodiesel; Carbon-based material; Esterification; Solid acid; Reusability
Abbreviations
-SO3H: Sulfonated groups; CMM: Carbon-based Microspheric Material; SCSAM: Sulfonated Carbon-based Solid Acid Microspheric Material; FESEM: Field-Emission Scanning Electron Microscope; TEM: Transmission Electron Microscope; FTIR: Fourier Transform Infrared Spectroscopy; TGA: Thermo gravimetric Analysis; DSC: Differential Scanning Calorimeter; BET: Brunauer-Emett-Teller; FFAs: Free Fatty Acids; GC: Gas chromatography; WCO: Waste cooking oils; FID: Flame ionization detector; FAME: Free Acid Methyl Ester; rpm: Revolutions per Minute
Introduction
The increasing demand for energy and depleting fossil fuel resources had led to the search for alternative fuels very imperative. Such alternative fuels must be technically feasible to be produced in large scale. They must also be economically competitive, environmental-friendly, and readily available. Biodiesel is a fuel consisting of alkyl esters derived from renewable lipid feedstock such as vegetable oil or animal fat [1]. Compared with regular diesel, biodiesel is biodegradable, the utilization of biodiesel as a fuel normally leads to lower CO2 and sulfur emissions and almost none particulate pollutants [2,3].
The most frequently used method for industrial scale biodiesel production is via transesterification reaction, which leads to a high yield of biodiesel in a very short time under the presence of base catalysts like NaOH and KOH [3]. But transesterification catalyzed by base catalysts requires raw oil to be of low Free Fatty Acids (FFAs) because the free fatty acids could react with the catalyst to produce soap, which makes the separation of product and unreacted raw oil very difficult.
However, biodiesel is not competitive compared with fossil fuels due to the high cost of raw material and cost of production [4]. One way to reduce the cost is to use cheap raw materials such as waste cooking oils (WCO) [5-7]. However, the use of WCO as the raw material for biodiesel manufacture is problematic due to its high free fatty acids (FFAs) content [8-10]. Therefore, the pretreatment of FFAs in WCO before transesterification is necessary, which is to convert the free fatty acids into methyl ester in the presence of homogeneous strong acid-catalysts, such as sulfuric acid and hydrochloric acid [11]. However, the use of liquid acids causes many problems, such as the difficulty in separating liquid acids from reaction medium, the formation of a large quantity of wastewater and the significant corrosion of equipment [12]. The use of heterogeneous catalysts is therefore preferred because it eliminates the need for the washing process and the catalysts can be easily recycled and reused. Other advantages of such include the simple downstream operations, better process economics and the yield of better quality biodiesel. Consequently, it is desirable to develop a highly active, inexpensive, green and reusable heterogeneous acid catalyst [13].
To date, many solid acid catalysts have been proposed to replace liquid acids for the esterification process, which include ion-exchange resin [7,10], acid zeolites [2,14], meso-structured silica functionalized with sulfonic groups, tungstated zirconia, sulfated zirconia, sulfonated polymers (Amberlyst-15), meso-porous materials [4,15- 17] etc. However, most solid-acid catalysts developed so far are expensive and involve complex synthetic procedures, which impede their commercialization in industrial scale production of biodiesel.
Recently, the synthesis of carbon-based solid acids and their application as heterogeneous catalysts in the production of biodiesel has attracted significant attention because carbon is a cheap and widely available material, and can be easily functionalized with functional groups [13,18-26]. Among them, SO3H-functionalized carbon microspheric material with regular spherical shapes and controllable sizes is attractive because of its metal-free, stable and recyclable nature. This catalyst has been used as a stable and highly active acid catalyst for various acid-catalyzed reactions such as esterification, hydrolysis and dehydration. To date, some methods have been developed for the synthesis of carbon microspheric materials. The hydrothermal method is commonly adopted for the preparation of carbon microspheric materials because of its simple steps and mild reaction conditions [27,28].
In this study, a sulfonated carbon-based solid acid microspheric material was prepared by using glucose as the raw material. This material was used as the catalyst in the esterification of oleic acid with methanol for the production of biodiesel. The structure and properties of the carbon material were characterized systematically. The catalytic performance (catalytic activity and reusability) of this carbon microspheric material was also investigated.
Materials and Methods
Materials
Chemicals used in this study, methanol, oleic acid, glucose and concentrated sulfuric acid were purchased from Sigma–Aldrich (USA). All the solvents and reagents used were either of HPLC grade or AR grade. All chemicals used were Analytical Reagent (AR) grade and were used without further purification.
Preparation of the SCSAM
The procedure for the preparation of sulfonated carbonbased solid acid microspheric material is listed in Figure 1. The hydrothermal carbonization of glucose started from about 9 g of glucose being dissolved in water (50 mL) to form a homogeneous aqueous glucose solution. It was then transferred to a stainless steel autoclave (80 mL capacity), in which the solution was heated up to 200 °C and kept isothermal for 4 hrs allow hydrothermal reactions to occur, which include polymerization reaction and carbonization reaction. Some aromatic compounds and oligosaccharides were formed in the polymerization step. In the carbonization step, carbon spheres covered by hydrophilic groups might arise from crosslinking induced by intermolecular dehydration of linear or branch like oligosaccharides. After the hydrothermal reactions, the reaction solution was cooled down naturally to room temperature. The black precipitate was collected by filtration, sequentially washed with boiling water, pure ethanol, and acetone to prepare Carbon-based Microspheric Material (CMM). The prepared sample was then dried in a vacuum at 60 °C for 24 h. About 1 g of the solid sample was then sulfonated by soaking in 60 mL concentrated sulfuric acid (98 %) at 200 °C under nitrogen atmosphere for 15 h. The sulfonated sample was collected by filtration, washed in succession with boiling water for 5-10 times, and then refluxed with toluene to remove unbonded polycyclic aromatic compounds. The filter cake was vacuumdried over night at 60 °C. The sulfonated carbon-based solid acid microspheric material was therefore prepared (Figure 1).
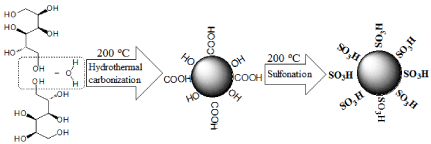
Figure 1: The illustration of the preparation method of SCSAM.
Catalysts characterization
Morphology of the samples prepared was inspected under a Zeiss Ultra plus Field-Emission Scanning Electron Microscope (FESEM) (Zeiss Co.) equipped with an energy dispersive X-ray (EDX) detector operated at an acceleration voltage of 10 kV. The samples were diluted with KBr and pressed into a thin wafer and the IR spectra were characterized by using a Fourier Transform Infrared Spectroscopy (FTIR) (TENSOR-37, Bruker Co.) by Attenuated Total Reflectance (ATR) in the wave number range of 4000-500 cm- 1. Textural properties of the calcined samples were measured based on N2 adsorption–desorption isotherms using a Micromeritics ASAP 2020M apparatus. The specific surface area (SBET) was calculated using the Brunauer–Emmett–Teller (BET) method. The thermal stability of the samples was investigated using a STA449 F3 Jupiter Thermo gravimetric analyzer (Netzsch Co.). The analysis was carried out in air atmosphere at a heating rate of 10 °C min-1 and was heated up from 50 to 900 °C. Total acidity of the catalysts, an indicator of the number of milli-equivalents of hydrogen ions (H+) in dry catalyst of unit quality [6], was obtained by using the KOH titration method. The reported values were the mean of at least five measurements and the average experimental error was ±5 %.
Catalytic activity test
Esterification of oleic acid with methanol (Scheme 1) was performed in a batch reactor (250 mL) equipped with a reflux condenser and a mechanical stirrer and operated at atmospheric pressure. The oleic acid was introduced first into the reactor and heated to the desired temperature. Then certain amount of methanol and catalyst were added into the reactor to allow esterification to occur. The primary reaction conditions were as following: oleic acid, 0.05 mol (equivalent to 15 g); methanol, 0.60 mol (equivalent to 19.2 g); catalyst loading 0.2 g (0.04 mmol H+ according to total acidity); mechanical stirring rate 360 rpm; reaction temperature, 65 °C; and reaction time, 8 h, except otherwise mentioned. The samples were taken out from the reactor every hour and the composition were tested using a Gas chromatography (GC7890B, Agilent Technologies) with a flame ionization detector (FID) equipped using a HP-5 column to determine the yield. The injector and FID detector were both operated at 250 °C. The oven was ramped from 50 to 250 °C at a rate of 10 °C min-1 and isothermal for 10 min. After completion, the reaction mixture was poured into a funnel and settled for 1 h to separate the excessive methanol and biodiesel. The biodiesel was further purified by decompression distillation to remove methanol and other impurities. After each run, the catalyst was filtrated from the reaction solution. It was then washed with ethyl ether twice to remove the adsorbed organic components and then washed twice with ethanol, boiling water, respectively. Afterward, the catalyst was dispersed in deionized water and was sonicated for another 30 min. The recovered catalyst was then dried at 50 °C in a vacuum oven for 24 h to completely remove residual water before reuse.
Scheme 1: The esterification of free fatty acid to free acid methyl ester (FAME).
Results and Discussion
Catalyst characterization
Morphology of the CMM and the SCSAM prepared were investigated by using SEM and the results are listed in Figure 2. It was clear that the carbon-based materials showed spherical structure with uniform size (about 2-3 μm) and smooth surfaces (Figure 2a). It was also noted in Figure 2b that the sulfonated carbon-based solid acid microspheric material showed similar spherical structure with the raw carbon-based microspheres, suggesting that the sulfonation process did not change the structure and the size of the carbonbased microspheres. The BET surface area of the two samples was 1.9 and 2.2 m²g-1, respectively, suggesting that both samples have no pores. The functional sulfonated groups together with carboxyl and hydroxyl groups were mainly on the external surface of the microspheres (Figure 1). This feature is beneficial for reactants to access the catalytic active sites and realize the esterification reaction (Figure 2).

Figure 2: SEM of CMM (a) and SCSAM (b).
The FTIR spectra of the CMM and the SCSAM are shown in (Figure 3). The two samples showed the same peaks associated with –OH vibration at 1260 cm-1 [29], C=C stretching vibration at 1630 cm-1 [30], C=O stretching vibration of the –COOH groups at 1730 cm-1 [31], the stretching vibration of O–H at 3430 cm-1 [32]. These peaks suggest that there were –OH, -COOH groups on both the CMM and the SCSAM, which is in good accordance with the preparation method illustrated in Figure 1. The two curves confirmed the presence of oxygen-containing groups during carbonization. However, a sharp peak appeared for the SCSAM at 1040 cm-1 [27], which was due to the symmetric O=S=O stretching vibrations of -SO3H groups. This suggested the presence of sulfonated groups in the SCSAM after sulfonation [28] (Figure 3).
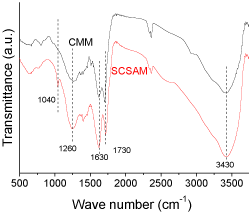
Figure 3: FTIR spectra of CMM and SCSAM.
TGA analysis and DSC analysis of the CMM and the SCSAM were also carried out in air atmosphere and the results are illustrated in (Figure 4a, and b). CMM shows excellent thermal stability, the weight loss only occurred at over 500 °C, which was assigned to the decomposition of graphene framework. This means that there were almost no functional groups in the raw CMM. However, there were mainly two weight loss peaks for the SCSAM from its TGA curve. The first weight loss below 450 °C (centering at 350 °C as shown in Figure 4 (b)) was the result of the decomposition of -SO3H and -COOH groups. The second peak over 500 °C was assigned to the decomposition of carbon frame work (Figure 4).
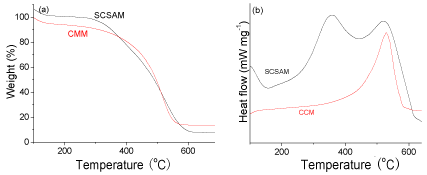
Figure 4: TGA (a) and DSC (b) curves of the CMM and SCSAM.
Catalytic performance of the SCSAM
The SCSAM was used in the esterification of oleic acid with methanol to evaluate their catalytic performance. The results are shown in (Figure 5 and Table 1). The results of esterification catalyzed by CMM, Amberlyst-15, H2SO4 and that without catalyst are also presented for comparison purpose. The yield was approximately 4.2 mol% when no catalyst was used and oleic acid itself acts as a weak acid catalyst. The CMM could slightly promote the esterification process to yield 27 mol% of biodiesel as it does not contain any catalytic active sites except for some weak acidic groups, such as -COOH. The SCSAM exhibited a better catalytic performance than Amberlyst-15, a widely used commercial cation exchange resin, although it had a higher total acidity (Table 1). This is mainly due to the fact that sulfonated microspheres contains abundant –COOH groups, which promote the esterification by adsorbing the water produced. The excellent catalytic performance is also associated with its smaller and uniform particle size (Figure 5).
Catalysts
Total acidity
(mmol g-1)a
SBET (m2 g-1)
Yield at 8 h (mol.%)
Blank
—
—
4.2
H2SO4
20.40
—
71.3
CMM
0.21
1.9
27.2
SCAMM
2.16
2.2
85.7
Amberlyst-15
4.9
30
58.2
aTotal acidity calculated by titration with NaOH, 0.1mol L-1.
Table 1: Catalytic activities on the esterification reaction by various catalysts (reaction conditions: oleic acid, 10 g; methanol/oleic acid molar ratio 12:1; catalyst loading 0.2 g (0.04 mmol H+); mechanical stirring, rate 360 rpm; reaction temperature, 65 °C; and reaction time, 8 h).
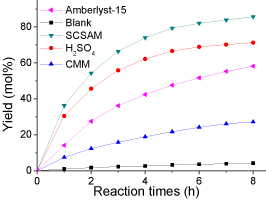
Figure 5: Esterification reaction catalyzed by different catalysts (reaction
conditions: oleic acid, 10 g; methanol, 20 g; catalyst loading 0.2 g; mechanical
stirring, rate 360 rpm; reaction temperature, 65 °C; and reaction time, 8 h).
Effect of methanol/oleic acid molar ratios on the esterification: To study the effect of methanol/oleic acid molar ratios on the esterification reaction, different experiments were carried out, in which the methanol/oleic acid molar ratios were in the range of 8:1 to 24:1. The results are listed in (Table 2). It can be seen that the yield increased from 66.7 to 86.2 mol% with the increase of the methanol/ oleic acid molar ratio from 8:1 to 12:1, equivalent to the increase of reactant concentration. However, the yield decreased from 86.2 to 74.3 mol% when the molar ratio increased from 12:1 to 24:1. This is mainly because the excessive methanol results in more methanol molecules being adsorbed on the catalyst surface and thus inhibits the formation of yield or even deactivates the catalyst. Thus the optimal methanol/oleic acid molar ratio was 12:1 in this reaction system.
Methanol /oleic acid molar ratio
8
12
16
20
24
Yield at 8h (mol.%)
66. 7
86.2
83.3
78.4
74.3
Table 2: Effect of methanol/oleic acid molar ratio (Reaction conditions: oleic acid 15 g, catalyst loading 0.2 g (0.04 mmol H+); mechanical stirring rate 360 rpm; reaction temperature, 65 °C; and reaction time, 8 h).
Effect of quantity of catalyst: Experiments were also carried out to evaluate how the yield was affected by the quantity of the SCSAM. The results were shown in (Table 3). The reaction did not proceed efficiently when the quantity of catalyst used was small. The reason was that there were not enough catalytic active sites for esterification reaction. The yield increased with the increase in catalyst loading within 0.25 g (0.05 mmol H+ according to the total acidity). When the catalyst loading exceeded 0.25 g, the yield didn’t increase with the increase in the quantity of catalyst used. Therefore, it can be concluded that the optimal catalyst loading quantity was 0.25 g (0.05 mmol H+).
Quantity of Catalyst (g)
0.1
0.15
0.2
0.25
0.3
Yield at 8 h (mol.%)
77.2
86.5
90.3
94.0
94.3
Table 3: Effect of quantity of catalyst amount (Reaction conditions: oleic acid 15 g, methanol 19.2 g; methanol/oleic acid molar ratio 12: 1; mechanical stirring rate 360 rpm; reaction temperature, 65 °C; and reaction time, 8 h).
Effect of reaction temperatures: The effect of reaction temperature on esterification is shown in (Figure 6). It was observed that the yield of biodiesel increased significantly with the increase of reaction temperature from 40 to 65 °C and the yield at 60 °C was almost the same as that of 65 °C. This is mainly because the increase in temperature could promote the motion of molecules and the rate of mass transfer. However, the yield decreased from 87 to 72 mol% when the temperature was further raised to higher than 70 °C. The reason was that when the reaction temperature exceeded the boiling point of methanol, methanol was vaporized and a large number of bubbles formed and thus inhibited the reaction. Therefore, it is reasonable to conclude that within the reaction conditions studied in this research, the optimal reaction temperature was 65 °C (Figure 6).
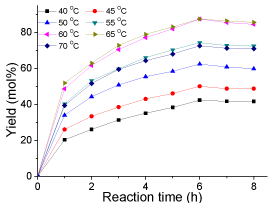
Figure 6: Effect of reaction temperatures(reaction conditions: oleic acid, 10
g; methanol, 20 g; catalyst loading 0.2 g; mechanical stirring, rate 360 rpm;
and reaction time, 8 h).
Effect of stirring: Normally, esterification reaction is a relatively slow process because esterification can only occur at the interface. So vigorous stirring is required to increase the contact area between the two immiscible reactants. To investigate the effect of the rate of mechanical stirring on biodiesel production, esterification was conducted at different stirring rates of 120, 240, 360 and 480 rpm, respectively. The results are shown in (Figure 7). It is clear that the yield was very low after 8 h when the rates of stirring were 120 and 240 rpm, under which the yields were only 74 and 81 mol%, respectively. However, the yield at 360 and 480 rpm were almost the same (over 90 mol%). Therefore, taking into account the production cost, the optimal stirring rate found in this study was around 360 rpm.
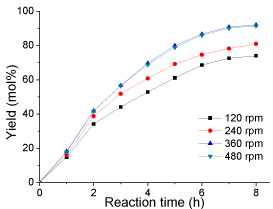
Figure 7: Effect of stirring rate on esterification(reaction conditions: oleic
acid, 10 g; methanol, 20 g; catalyst loading 0.2 g; reaction temperature, 65
°C; and reaction time, 8 h).
Effect of reaction time: The effect of reaction time on esterification was studied by prolonging reaction time to 12 h. The results are illustrated in (Figure 8). The esterification catalyzed by sulfuric acid could be divided into three stages: in the first stage (0-4 hours), the yield increased significantly from 0 to 70 mol% at a very high reaction rate; in the second stage (4-8 hours), the reaction rate decreased significantly and the yield reached about 80 mol%, in the last stage (8-12 hours), the yield kept stable at 80 mol%. However, the esterification by the SCSAM exhibited different behaviors compared with the esterification by sulfuric acid, which could only be divided into two stages: in the first stage (up to 8 hours), the esterification yield reached 95 mol% at a very high reaction rate; in the second stage (over 8 hours), the yield remained constant. Taking into account the production cost and the yield, the optimal reaction time found in this study was 8 h when the SCSAM material was used as the catalyst.
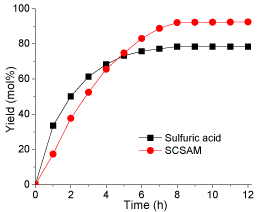
Figure 8: Effect of reaction time(reaction conditions: oleic acid, 10 g;
methanol, 20 g; catalyst loading 0.2 g; mechanical stirring, rate 360 rpm;
reaction temperature, 65 °C).
Reusability of the SCSAM
Generally, apart from the catalytic activity, the reusability of catalyst is of great influence on production cost. In order to investigate the reusability of the sulfonated carbon-based solid acid microspheric material, the spent catalyst was collected, washed with water and employed in esterification for six runs under the optimal reaction conditions. The results of the recycled catalyst are shown in (Figure 9). The optimal reaction conditions determined in this study were: methanol/oleic acid molar ratio 12:1, catalyst loading 0.25 g (0.05 mmol H+), reaction temperature 65 °C, reaction time 8 h and mechanical stirring rate 360 rpm. It can be seen that the SCSAM showed good catalytic activity at the first run, with a yield of 92 mol%. A gentle deactivation occurred after the first run. The yield was still approximate 84 mol% after five runs. This gentle deactivation is mainly attributed to the loss in total acidity due to the leaching of active sites, the sulfonated groups. However, it is still reasonable to conclude that reusability of the SCAMM was relatively high and the catalyst remained high catalytic performance after having been used for 6 runs.
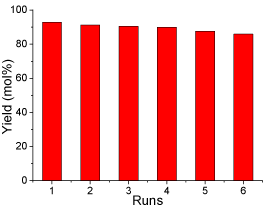
Figure 9: The catalytic performance of the SCSAM versus recycling
times(reaction conditions: oleic acid, 10 g; methanol, 20 g; methanol/oleic
acid molar ratio 12:1,catalyst loading 0.25 g; mechanical stirring, rate 360
rpm; reaction temperature, 65 °C; and reaction time, 8 h).
Conclusion
In conclusion, a sustainable route was developed for the synthesis of sulfonated carbon-based solid acid microspheric material for biodiesel production using glucose as the raw materials, in which the sulfonated groups were attached to the carbon microspheres. The catalyst developed showed high catalytic performance in the esterification of oleic acid with methanol. Such high catalytic performance was attributed to the sulfonated microspheres, which had uniform particle size and better affinity to the reactants. The SCSAM also showed good reusability, with only a gentle decrease in catalytic efficiency even after 5runs.This catalyst is green, noncorrosive, and renewable, and has the potential to be used in industrial production of biodiesel.
Acknowledgement
Following funding bodies are acknowledged for partially sponsored this piece research: Ningbo Bureau of Science and Technology (Innovation Team Scheme, 2012B82011 and Major Research Scheme, 2012B10042) and Ministry of Science and Technology (International Science & Technology Cooperation Programme, 2012DFG91920). The University of Nottingham is acknowledged for providing scholarship to the first author.
References
- Ma F, Hanna MA. Biodiesel production: a review. Bioresour Technol. 1999; 70: 1-15.
- Narkhede N, Patel A. Biodiesel Production by Esterification of Oleic Acid and Transesterification of Soybean Oil Using a New Solid Acid Catalyst Comprising 12-Tungstosilicic Acid and Zeolite Hβ. Ind Eng Chem Res. 2013;52: 13637-13644.
- López DE, James G. Goodwin J, Bruce DA. Transesterification of triacetin with methanol on Nafion® acid resins. J Catal. 2007; 245: 381-391.
- Dhainaut J, Dacquin J-P, Lee AF, Wilson K. Hierarchical macroporous–mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2009; 12: 296-303.
- Zhang H, Ding J, Zhao Z. Esterification of different FFAs with methanol by CERP/PES hybrid catalytic membrane for biodiesel production. Journal of Central South University. 2012; 19: 2895-2900.
- Zhang H, Ding J, Qiu Y, Zhao Z. Kinetics of esterification of acidified oil with different alcohols by a cation ion-exchange resin/polyethersulfone hybrid catalytic membrane. Bioresour Technol. 2012; 112: 28-33.
- Zhang H, Ding J, Zhao Z. Microwave assisted esterification of acidified oil from waste cooking oil by CERP/PES catalytic membrane for biodiesel production. Bioresour Technol. 2012; 123: 72-77.
- Birla A, Singh B, Upadhyay SN, Sharma YC. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour Technol. 2012; 106: 95-100.
- Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, Wang J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energy 2010; 87: 2589-2596.
- Özbay N, Oktar N, Tapan NA. Esterification of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel. 2008; 87: 1789-1798.
- DeSimone JM. Practical Approaches to Green Solvents. Science. 2002; 292: 799-803.
- Ji J, Zhang G, Chen H, Wang S, Zhang G, Zhang F, et al. Sulfonated graphene as water-tolerant solid acid catalyst. Chem Sci. 2011; 2: 484-487.
- Hara M, Yoshida T, Takagaki A, Takata T, Kondo JN, Hayashi S, et al. A carbon material as a strong protonic acid. Angew Chem Int Ed. 2004 May 24; 43: 2955-2958.
- Žilková N, Bejblová M, Gil B, Zones SI, Burton AW, Chen C-Y, et al. The role of the zeolite channel architecture and acidity on the activity and selectivity in aromatic transformations: The effect of zeolite cages in SSZ-35 zeolite. J Catal. 2009; 266: 79-91.
- Pirez C, Lee AF, Manayil JC, Parlett CMA, Wilson K. Hydrothermal saline promoted grafting: a route to sulfonic acid SBA-15 silica with ultra-high acid site loading for biodiesel synthesis. Green Chem. 2014; 16: 4506-4509.
- Pirez C, Caderon J-M, Dacquin J-P, Lee AF, Wilson K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012; 2: 1607-1614.
- Pesaresi L, Brown DR, Lee AF, Montero JM, Williams H, Wilson K. Cs-doped H4SiW12O40 catalysts for biodiesel applications. Appl Catal, A 2009; 360: 50-58.
- Okamura M, Takagak A, Toda M, Kondo JN, Domen K, Tatsumi T, et al. Acid-Catalyzed Reactions on Flexible Polycyclic Aromatic Carbon in Amorphous Carbon. Chem Mater. 2006; 18: 3039-3045.
- Takagaki A, Toda M, Okamura M, Kondo JN, Hayashi S, Domen K, et al. Esterification of higher fatty acids by a novel strong solid acid. Catal Today 2006; 116:157-161.
- Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, et al. Biodiesel made with sugar catalyst. Nature. 2005; 438.
- Suganuma S, Nakajima K, Kitano M, Hayashi S, Hara M. sp3-Linked Amorphous Carbon with Sulfonic Acid Groups as a Heterogeneous Acid Catalyst. ChemSusChem 2012; 5: 1841-1846.
- Fukuhara K, Nakajima K, Kitano M, Kato H, Hayashi S, Hara M. Structure and Catalysis of Cellulose-Derived Amorphous Carbon Bearing SO3H Groups. ChemSusChem 2011; 4: 778-784.
- Hara M. Biomass conversion by a solid acid catalyst. Energy Environ Sci. 2010; 3: 601-607.
- Hara M. Biodiesel Production by Amorphous Carbon Bearing SO3H, COOH and Phenolic OH Groups, a Solid Brønsted Acid Catalyst. Top Catal. 2010; 53: 805-810.
- Yamaguchi D, Hara M. Starch saccharification by carbon-based solid acid catalyst. Solid State Sci 2010; 12: 1018-1023.
- Yamaguchi D, Kitano M, Suganuma S, Nakajima K, Kato H, Hara M. Hydrolysis of Cellulose by a Solid Acid Catalyst under Optimal Reaction Conditions. The Journal of Physical Chemistry C. 2009; 113: 3181-3188.
- Liang X, Zeng M, Qi C. One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon. 2010; 48: 1844-1848.
- Macia-Agull JA, Sevilla M, Maria A. Diez, Fuertes AB. Synthesis of Carbon-based Solid Acid Microspheres and Their Application to the Production of Biodiesel. ChemSusChem. 2010; 3: 1352-1354.
- Daniela C Marcano, Dmitry V Kosynkin, Jacob M Berlin, Alexander Sinitskii, Zhengzong Sun, Alexander Slesarev, et al. Improved synthesis of graphene oxide. ACSnano. 2010; 4: 4806-4814.
- Mazzotta MG, Gupta D, Saha B, Patra AK, Bhaumik A, Abu-Omar MM. Efficient solid Acid catalyst containing lewis and bronsted Acid sites for the production of furfurals. ChemSusChem. 2014; 7: 2342-2350.
- Zhu M, He B, Shi W, Feng Y, Ding J, Li J, et al. Preparation and characterization of PSSA/PVA catalytic membrane for biodiesel production. Fuel. 2010; 89: 2299-2304.
- Liu XY, Huang M, Ma HL, Zhang ZQ, Gao JM, Zhu YL, et al. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules. 2010; 15: 7188-7196.