
Research Article
Austin Chem Eng. 2017; 4(1): 1049.
Studies on the Removal of Pb(II) from Aqueous Solutions by Adsorption with E. Globulus Leaf Powder through Response Surface Methodology
Hymavathi D and Prabhakar G*
Department of Chemical Engineering, S.V. University College of Engineering, Tirupati, Andhra Pradesh, India
*Corresponding author: Garimella Prabhakar, Department of Chemical Engineering, S.V. University College of Engineering, Tirupati, Andhra Pradesh, India
Received: April 12, 2017; Accepted: May 17, 2017; Published: May 24, 2017
Abstract
An exhaustive batch experimental investigation to treat lead-laden waters by adsorption using leaf powder of Eucalyptus globulus is reported. Based on full factorial approach, optimum conditions to yield a removal of 96.58% are identified as initial ion concentration of 20mg/L, sorbent dosage 25g/L at a pH of 5.0 and a temperature of 303K. Freundlich model adequately represents the equilibrium and the maximum adsorption capacity is found to be 6.803mg/g. The process is endothermic and spontaneous. Adsorption follows second order kinetics. The adsorption process compares well with other similar studies.
Keywords: Pb(II) removal; Adsorption; E. globules; Equilibrium; Kinetics; RSM
Nomenclature
Co: Initial Concentration of Pb(II) solution (mg/L); Ce: Equilibrium Concentratin of Pb(II) solution (mg/L); qe: Amount Sorbed Per Unit Weight Of Biosorbent At Equilibrium (mg/g); qet: Equilibrium Metal Uptake Capacity At Time (mg/g); qmax: Maximum Metal Uptake Capacity (mg/g); qt: Metal Uptake Capacity At Time t (mg/g); Kdiff: Intra-Particle Diffusion Rate Constant; c: Thickness Of Boundary Layer; m: Mass Of Adsorbent (g); w: Adsorbent Dosage (g/L); V: Volume of the Pb(II) solution (L); b=kL: Affinity Constant Or Energy To Biosorption (L/g); n: number(dimensionless); Kf : Freundlich coefficient (mg/g); K1 : First Order Equilibrium Rate Constant (1/min); K2: Second Order Equilibrium Rate Constant (g/mg.min); AT: Temkin Adsorption Intensity, (L/g); AE: Elovich constant; BE: Initial Adsorption Rate; E: Mean Free Energy Of Sorption Per Of The Molecule Of Sorbate; bT: Heat Of Adsorption Constant
Abbreviations
R: Universal Gas Constant (8.314mol-1K-1); X1: Initial Concentrations of Pb(II) (mg/L); X2: pH(dimensionless); X3: Adsorbent Dosage (g/L); X4: Absolute Temperature (K); RSM: Response Surface Methodology; ANOVA: Analysis Of Variance; Max: Maximum Metal Uptake Capacity; Diff: Intra-Particle Diffusion Rate Constant
Introduction
Water contamination is mainly due to the accumulation of heavy metals, from different industries like metallurgical, battery, electroplating and metal finishing industries, chemical manufacturing and tanneries [1,2]. Among heavy metals viz., Co, Cr, Zn, Cd, Cu, Pb, Hg, As, Al and Ni, Pb(II) is one of the most toxic ion causing serious health issues related to liver damage, nervous system, kidneys, reproductive system, neurological activity and also causes high hypertension [3,4]. Maximum allowable concentration for Pb(II) ion limit value of 0.01mg/L in drinking water recommended by World Health Organization [5] and the permissible level of Pb(II) in wastewater is 0.05mg/L given by the Environmental Protection Agency (EPA) [6]. Conventional methods - chemical precipitation [7], reverse osmosis [8], ion exchange [9], coagulation [10], electro dialysis [11] and ultra filtration [12] were studied earlier. These methods were found to be not so promising due to incomplete metal removal, high reagent and high energy requirements.
Adsorptive treatment of lead waters was probed with different types of waste materials and a few of them are as follows. Nile rose plant [13], chaff [14], rice husk [15], coir fiber waste [16], banana stems [17], wheat bran[18], coffee grounds [19], tree ferns [20], palm kernel fibres [21], crop milling waste-black gram husk [22], pomegranate peels [23] , peanut skins [24], cone biomass of Pinus sylvestris [25], carbon derived from agricultural waste[26], almond shell [27], native and chemically treated olive stone [28], residue of all spice [29], ceder leaf ash [30], cashew nut shell [31], Peanut shell [32], native and chemically treated olive tree pruning [33], pine cone shell [34] and rapeseed biomass [35] were used in the studies and Eucalyptus globulus , leaf powder of cheap and widely available which is preferred as a natural antimicrobial agent, industrial solvent, and deodorant, is tried for the removal of lead from aqueous systems [36,37].
In present work, E. globules L., used as an adsorbent for removal of Pb(II) from aqueous solution and E. glubulus belongs to the family of myrtaceae. Eucalyptus bark was used for the removal of chromium [38] and mercury [39]. Till –to- date, non literature is available on E. globulus leaf powder for removal of Pb(II).
Materials and Methods
Preparation of stock solution
All chemical compounds used are of analytical grade (Merck).
A Stock solution of 500ppm Pb(II) is prepared by dissolving 0.4055mg of 98.5% pure Pb(NO3)2 in 500ml of distilled water. It is diluted to different levels, appropriate to the study. pH of the solution is adjusted using 0.1N NaOH and 0.1N H2SO4. Final Pb(II) ion concentration is obtained by Inductively Coupled Plasma Optical Emission Spectroscope (Perkin Elmer model Optima 8000). FTIR (ALPHA interferometer (ECO-ATR)), Bruker, Germany) in the range of 4000 – 500 cm-1 is employed to identify the functional groups that are involved in adsorption. Elemental composition is recorded by Scanning Electron Microscope, (SEM–EVO MA 15) with Electron Dispersive X- Ray Spectroscope of OXFORD INSTRUMENTS (Inca Penta FET x3).
Preparation and activation of biosorbent
E. globulus leaves are collected in the University Campus, water washed thoroughly to clear the surface impurities, and is then sun dried. They are grounded into a fine powder and 63μm – size particles are collected. The particles are further washed with water to remove coloring agents, dried at room temperature and are stored in air tight bottles for further studies.
Biosorption studies
Sufficient numbers of flasks, each containing 50ml solution of 20mg/L Pb(II) are taken and the solution pH adjusted. A known quantity of adsorbent is added and the flasks are agitated at constant speed on a shaker at room temperature, Flasks are withdrawn at suitable time intervals, the content filtered and Pb(II) estimation in the liquid sample is made. Similarly, the procedure is repeated with different quantities of adsorbent and other parameters to make the study complete. Percentage removal Pb(II) is calculated using the formula
The equilibrium metal uptake capacity is estimated by using
Response surface methodology (C.C.D) and optimization of Pb(II) removal
RSM is a group of mathematical and statistical techniques for modeling and analysis of problems in which the response of a model is influenced by several variables [40]. A 24 full-factorial design, 6 center points and 8 axial points leading to 30 experimental runs is performed to study the effect of the four contributing parameters using statistical software, Design expert 10.0.03 (Ease state, USA). Central Composite Design (CCD) consists of 2n factorial runs with 2*n axial runs and the minimum number of experiments that need be conducted is provided by equation 3.
No of experiments (N) = 2n+2n+6 (center points) = 2*4+2*4+6=30 (3)
Each variable is investigated at two levels and as the number of factors/operating variables increases, then the number of experimental runs for complete picture also increases.
Y=f (X1, X2, X3 …Xn) (4)
In the present case, four factors - initial metal ion concentration (X1), initial solution pH (X2), adsorbent dosage (X3) and temperature of solution (X4) are selected as independent variables in equation 4 and by fixing the contact time and size of the adsorbent, the percentage removal of Pb(II) is obtained. Percentage adsorption (%Y) is considered as the dependent variable and the experimental design made with range and levels (-2, -1, 0, 1, 2) of independent variables. In the optimization process, the response can be related to the independent variables by quadratic (second degree) equation and the model equation is given in equation 5.
(5)
where Y is estimate response of the system, β0 is constant coefficient, β1, β2, β3 and β4 are linear coefficients, β12, β13, β14, β23, β24 and β34 are interaction coefficients among the four factors, β11, β22, β33 and β44 are quadratic coefficients, X1, X2, X3 and X4 are independent variables. A multiple regression analysis is then performed to obtain the values of the coefficients. A total of 30 experiments are needed to estimate the biosorption of Pb(II) on to E. globulus L. The responses and corresponding parameters are modeled and optimized using analysis of variance (ANOVA) and by the correlation coefficient (R2). The R2 value shows a measure of how variability in the observed response values can be simplified by experimental factors and their interactions [41].
Results and Discussion
Optimization using response surface methodology (RSM)
It is pointed out that six factors are critical in adsorption. The smaller the particle, the higher is the surface available for transfer and the higher the transfer. However, post adsorption separation puts a limit on the particle size. In the present study a particle of 63μm is used. The time progress of adsorption is studied first and the process has taken 60 minutes to reach equilibrium, Thus, keeping 63μm particle size and 60 minutes of contact time fixed, the effect of other vital parameters, namely, Pb(II) ion concentration, initial solution pH, sorbent dosage and temperature is studied. A total of 30 experimental runs are conducted and the results of the experimentation are in (Table 1). Levels of different process variables in coded and uncoded form for adsorption of Pb(II) using E. globulus L. leaf powder in (Table 2). Thus, 96.58% removal of Pb(II) could be achieved, when 20mg/L Pb(II) is treated with 25g/L E. globulus L. at a pH of 5.0 and temperature of 303K. The interactive contributions of the variables are further studied with model expression given as equation 3.
Run
X1(Co)
X2(pH)
X3 (w)
X4(T)
% Adsorption of Pb(II)
Experimental
Predicted
1
-2
0
0
0
94.42
94.4391
2
1
-1
1
1
95.34
95.3214
3
-1
-1
-1
1
96.01
95.9773
4
0
0
-2
0
95.7
95.733
5
0
0
0
-2
94.42
94.4155
6
-1
1
-1
1
92.42
92.4071
7
-1
1
1
-1
94.77
94.7846
8
0
0
0
0
96
96
9
-1
-1
1
1
95.881
95.8978
10
-1
1
-1
-1
93.158
93.1381
11
0
0
2
0
96.58
96.6018
12
1
1
-1
-1
93.634
93.601
13
1
1
-1
1
93.43
93.4143
14
0
0
0
0
96
96
15
1
1
1
-1
94.555
94.5493
16
0
-2
0
0
95.53
95.5361
17
0
0
0
0
96
96
18
0
2
0
0
92.36
92.4086
19
-1
-1
-1
-1
96.19
96.2035
20
1
-1
-1
1
96.13
96.0991
21
1
-1
-1
-1
95.77
95.7811
22
0
0
0
0
96
96
23
0
0
0
2
94.84
94.8993
24
2
0
0
0
94.29
94.3256
25
-1
-1
1
-1
95.25
95.2273
26
-1
1
1
1
95
94.9504
27
1
-1
1
-1
94.11
94.1066
28
0
0
0
0
96
96
29
1
1
1
1
95.289
95.2593
30
0
0
0
0
96
96
Table 1: Result from CCD for Pb(II) Adsorption by E. globulus L.
Variable
Name
Range and level
-2 -1 0 1 2
X1
Initial concentration(Co) mg/L
10 15 20 25 30
X2
pH of the solution
3 4 5 6 7
X3
Biosorbent dosage(w), g/L
5 10 15 20 25
X4
Temperature(T), K
283 293 303 313 323
Table 2: Levels of different process variables in coded and uncoded form for Adsorption of Pb (II) using E. globulus L. leaf powder.
The equation 6 suggests that a metal ion concentration (Co) = 20.904 mg/L, pH = 5. 258, sorbent dosage (w) = 15.372g/L, and temperature (T) = 302.006K yield the highest removal 97.087% and there is good agreement between experimental values and those, calculated with the model equation. A comparison of optimal values (predicted) and the percentage removal (actual), and the normal plot for residual is in (Figure 1(i)). Interactive contributions of the four variables - Pb(II) ion concentration, initial solution pH, adsorbent dosage and temperature, are presented in surface contour plots in (Figure 1(ii)) for better understanding. The ANOVA and regression coefficient values are presented as (Table 3).
Source
Sum of
Squares
df
Mean
Square
F
Value
p-value
Prob > F
Model
38.15
14
2.72
2256.97
< 0.0001
significant
X1-Initial Concentration
0.019
1
0.019
16
0.0012
X2-pH
14.67
1
14.67
12152.03
< 0.0001
X3-Biosorbent dosage
1.13
1
1.13
937.84
< 0.0001
X4-Temperature
0.35
1
0.35
290.83
< 0.0001
X1 *X2
0.78
1
0.78
649.07
< 0.0001
X1 *X3
0.49
1
0.49
403.82
< 0.0001
X1 *X4
0.3
1
0.3
245.34
< 0.0001
X2*X3
6.88
1
6.88
5697.4
< 0.0001
X2*X4
0.25
1
0.25
211.02
< 0.0001
X3*X4
0.8
1
0.8
666.05
< 0.0001
X12
4.49
1
4.49
3715.37
< 0.0001
X22
7.05
1
7.05
5837.42
< 0.0001
X32
0.048
1
0.048
39.78
< 0.0001
X42
3.09
1
3.09
2559.5
< 0.0001
R2 (Adj) = 0.9991 and R2 (Pred) = 0.9973
Table 3: ANOVA and estimated regression coefficients for the Pb (II) biosorption onto E. globulus L.
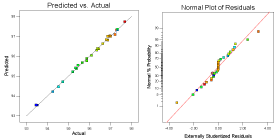
Figure 1(i): A comparison of predicted values and the percentage removal
(Actual values) and the normal plot for Residual for Pb(II) onto E. globulus. L.
The Model F-value of 2256.97 in (Table 3) implies that the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. All the terms with F< Probability <0.05 are significant. In this case all model terms X1, X2, X3, X4, X1*X2, X1*X3, X1*X4, X2*X3, X2*X4, X3 *X4, X1 2, X2 2, X3 2 X4 2 are significant. A value greater than 0.05 indicate the model terms are not significant.
Isotherm studies
Four different standard, the two-parameter adsorption isotherm models are examined for suitability to represent equilibrium, concerning Pb(II) removal.
Langmuir isotherm model [42]
Feasibility of the Langmuir isotherm depends on dimensionless constant is expressed by separation factor, RL.
In the present study the value RL is 0.913, indicates a favorable condition.
Freundlich isotherm model [43]
Temkin isotherm model [44]
Dubinin–Radushkevich isotherm model
Sorption energy is calculated by Dubinin–Radushkevich [45] isotherm model to predict the nature of adsorption process, i.e., physical or1 chemical. The linear from of the model is described as.
The Polanyi potential [46] which is equal to,
The plot of lnqe against e2 gave a straight line from which the values of K and qD, where qD is the Dubinin-Radushkevich isotherm constant related to the degree of sorbate sorption by the sorbent surface. From the K value, the mean sorption energy (E) is evaluated as
The E (kJ/mol) value gives the information regarding type of adsorption, physical or chemical. If E < 8kJ/mol, the adsorption process is physical in nature and in the 8–16 kJ/mol range and it is chemical in nature [47]. In the present case, the adsorption is in chemical in nature. The parametric values of these models are given in (Table 4) and represent the system well at equilibrium, with Freundlich model being marginally superior to the other models.
Isotherm Models
Equation
Parameters
R2
Langmuir
ce/qe = 0.147ce+0.885
qmax=6.803mg/g
kL=b=0.166L/g
0.9486
Freundlich
lnqe = 0.5724lnce + 0.047
Kf=1.046mg/g
n=1.75
0.9918
Temkin
qe= 1.340 lnce+1.033
bT=1879.95 kJ/mol
AT=2.162 K L/g
0.9492
D-R model
lnqe=-3E-07e2+1.2743
K=3e-7
qm=3.576mg/g
E=13.519 kJ/mol
0.8446
Table 4: Isotherm equations and constants at optimum conditions.
Thermodynamics of adsorption
Adsorption process is temperature dependent. A plot of 1/T against the ratio of equilibrium concentrations of Pb(II) ion in liquid and solid phases is plotted on a semi logarithmic coordinate system and the plot is as shown in (Figure 2). Changes in enthalpy, Gibb’s free energy and entropy are calculated to explore the nature of the adsorption, using the following equations 12 and 13.
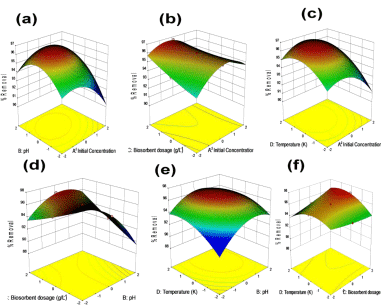
Figure 1(ii): Surface contour plots, (a). pH -Initial conc –% Removal, (b).
Initial conc– dosage – % Removal, (c). Temp. – Initial conc –% Removal,
(d). pH- dosage -% Removal, (e). pH – Temp. – % Removal, (f). Temp.
Dosage – % Removal.
Thermodynamic parameters are evaluated through van’t Hoff plot (Figure 2) and the values are ΔH = 18.0664kJ/mole, ΔG= (-) 0.76kJ/ mole and ΔS= 62.131J/ mole -K. It is evident from the property values that the adsorption is endothermic and spontaneous.
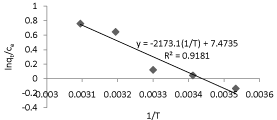
Figure 2: van’t Hoff plot for biosorption of Pb(II).
Pb(II) sorption kinetics
Chemical interactions, between the adsorbate and the adsorbent, are described by the different kinetic expressions, in the literature and their applicability to the present case is studied.
Pseudo 1st-order model [48],
Pseudo 2nd-order model [49],
Elovich model [50]
Intra-particle diffusion model [51]
A plot of log (qe-qt) against time (t) yielded an intercept different from experimentally determined qe, hence, pseudo first order equation does not represent the adsorption. Pseudo second order model considers the rate-limiting step as the formation of chemisorptive and physiorption bond, involving sharing of electrons between the solute and sorbent. This pseudo-second-order kinetic model, describes the adsorption process with an R2 of 0.997. The other three expression exhibit an inferior fit to the data, with lower R2. The values of kinetic parameters and the regression coefficient are as shown in (Table 5).
Kinetic model
Equation
Parameters
R2
Pseudo1st order
log(qet–qt) = - 0.027t-0.665
K1=0.0306min-1
0.959
Pseudo 2nd order
(t/ qt) = 0.511 + 0.434
K2=0.602 g/mg.min
1
Elovich model
qt=0.069 lnt+1.6555
AE=14.492
BE=1.8*109
0.9577
Intra-particle diffusion
qt=0.0261t1/2+1.8222
Kdif = 0.0261
C=1.7411
0.9009
Table 5: The rate equations and coefficients for adsorption of Pb (II) on to E globulus L.
FTIR characterization
FTIR spectra to characterize the functional groups, which are responsible for adsorption process, before and after adsorption of Pb (II), are as shown in (Figure 3(a1-a2)) and as in (Table 6). As can be seen from the (Table 6), ester functional group are present after adsorption process, C-H bending and –N-H stretch are not involved in metal uptake and hence are not affected(shift is zero). Other functional groups show shifts, both on positive and negative sides, indicating their relative role in metal capture. -C-H stretch, N-H and O-H stretch and skeletal vibration of the C-O stretch is the prime functional group that is involved in metal ion loading. C=O stretch in aldehyde group are present after adsorption. C- Cl stretching vibrations and C-Br stretch in alkyl halide are not found after adsorption.
Functional group
E. globulus L.
Before adsorption
After adsorption
Shift
Esters impurities
3826-3716
N-H and O-H stretch
3327
3336
9
(3200-3500)
strong O-H stretch
3249
3244
-5
C-H stretch
2911
2920
-9
alkyne triple bonds and nitrile triple bonds
2354
2353
-1
C=O stretch in aldehyde group
----
1718
C=C stretch
1628
1633
5
N-O stretch in Nitro group
------
1524
-C-H bending
1441
1441
0
C-N stretch
1317
1320
3
(amide III) modes of the residual N-acetyl groups
C-N stretch
1221
1218
-3
C-N stretch
1153
1153
0
skeletal vibration of the C-O stretch
1046
1055
11
C-Cl stretching vibrations
771
-----
C-Br stretch in alkylhalide
582
----
Table 6: Wave number (cm-1) for major peak from FT-IR analysis for Pb (II) adsorption onto E. globulus L.
Adsorbent
Concentration
Time
pH
Metal uptake, mg/g
References
(mg/l)
min
Ficus religiosa leaves
10-1000
15
4
37.45
[53]
Bael leaves (Aegle marmelos)
8.7-180.2
30
5.1
104
[54]
Cinnamomum camphora leaves
50-400
60
5
73.15,
[55]
Black cumin
20-May
60
5.1
8.08
[56]
Maize bran
100-150
100
6.5
142.86
[57]
Tephrosia purpurea
25–200
130
5.4
100
[58]
Phaseolus vulgaris L
40–80
20
5
42.68
[59]
Sargassum ilicifolium
20-200
120
3.7
195
[60]
E.globulus L
20-150
60
5
6.803
Present study
Table 7: Comparison of maximum biosorption capacity based on the Langmuir isotherm by different adsorbents for Pb(II) from aqueous solution.
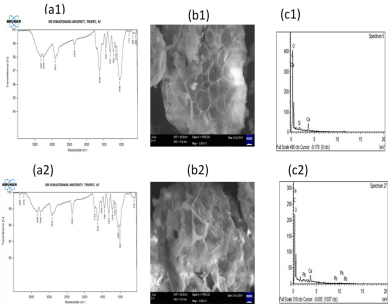
Figure 3(a1-a2): FTIR spectra, (b1- b2). SEM images and (c1- c2). EDAX
spectra of the E. globulus L. before and after Pb(II) loading.
SEM with EDAX analysis
Scanning Electron Microscopy (SEM) is used to verify the morphological differences between the Pb(II) free and Pb(II) loaded E. globulus L. The surface character is as shown in (Figure 3(b1-b2)) and from this image E. globulus L. exhibits an altered surface after loading of Pb(II) a similar report is available in the literature [52].
The elemental composition of the adsorbent (before and after adsorption) is analyzed by Energy Dispersive Analysis System (EDAX). The EDAX spectrum for E. globulus L. powder shown in (Figure 3(c1)) indicates the presence of only O, C, Ca and Si, but no Pb(II) ions on the surface of E. globulus L. before adsorption. After adsorption, spectra include a characteristic peak for Pb(II) at 2.5keV, as shown in (Figure 4(c2)), confirming the metal ion loading.
Comparative study
The outcome of the present investigation compared against similar attempts is given in (Table 6) and from the table, it can be concluded that the performance of E. globulus L. is reasonable good.
Conclusion
E. globulus L., an agricultural byproduct and is easily available. This work demonstrates that it used as an adsorbent for the removal of Pb(II) ions from aqueous solution. Effect of operating parameters viz. metal ion concentration, initial solution pH, sorbent dosage and temperature is probed and at optimum conditions, the removal of Pb(II) is found to be 96.58% (qmax 6.803mg/g). The equilibrium data fits well to the Freundlich model. The absorption is endothermic and negative values of ΔG confirm an affinity between E. globulus L and Pb(II).
References
- Demirbas E, Kobya M, Oncel S, Sencan S. Removal of Ni(II) from aqueous solution by adsorption onto hazelnut shell activated carbon: equilibrium studies. Biores Technol. 2002; 84: 291–293.
- Pastircakova K. Determination of trace metal concentrations in ashes from various biomass materials. Energy Edu Sci Technol. 2004;13: 97–104.
- Shud D, Mahajan G, Kaur MP. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solution – A review. Bioresour Technol. 2008; 99: 6017- 6027.
- Dursun AY. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. J Biochem Eng. 2006; 28: 187–195.
- Lalhruaitluanga H, Jayaram K, Prasad MNV, Kumar KK. Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo) – a comparative study. J Hazard Mater. 2000; 175: 311-318.
- Goel J, Kadirvelu K, Raja gopal C, Garg V K. Removal of lead(II) by adsorption using treated granular activated carbon: batch and column studies. J Hazard Mater B. 2005; 125: 211–220.
- Gray NF. Water technology: An introduction for environmental scientists and engineers. 2ndedn. Ireland: Elsevier Sci and Technol Books. 2005.
- Shahalam AM, Ali AH, Zawhry AA. Feed water pretreatment in RO systems in the Middle East. Desal. 2002; 150: 235-245.
- Kang SY, Lee JU, Moon SH, Kim KW. Competitive adsorption characteristics of Co(II), Ni(II) and Cr(III) by IRN-77 cation exchange resin in synthesized waste water. Chemospere. 2004; 56: 141-147.
- Samrani AGE, Lartiges BS, Villieras F. Chemical coagulation of combined sewer over flow: Heavy metal removal and treatment optimization. Water Res. 2008; 42: 951- 960.
- Sadrzadeha M, Mohammadi T, Ivakpour J, Kasiri N. Neural network modeling of Pb(II) removal from wastewater using electro dialysis. J Chem Eng Pro. 2009; 48: 1371-1381.
- Aguirre JL, Garcia V, Pongracz E, Keiski RL. The removal of zinc from synthetic waste waters by micellar-enhanced ultrafiltration: Statistical design of experiments. Desal. 2009; 240: 262-269.
- Abdel-Halim SH, Shehata AMA, El Shahat MF. Removal of lead ions from industrial waste water by different types of natural materials. Water Res. 2003; 37: 1678–1683.
- Han R, Zhang J, Zou W, Shi J, Liu H. Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater. 2005; 125: 266–271.
- Zulkali MMD, Ahmad AL, Norulakmal, NH. Oryza sativa L. husk as heavy metal adsorbent: Optimization with lead as model solution. Bioresour. Technol. 2006; 97: 21–25.
- Shukla SR, Pai RS. Comparison of Pb(II) uptake by coir and dye loaded coir fibres in a fixed bed column. J Hazard Mater. 2005; 125: 147– 153.
- Noeline BF, Manohar DM, Anirudhan TS. Kinetic and equilibrium modeling of lead(II) sorption from water and wastewater by polymerized banana stem in a batch reactor. Sep Purif Technol. 2005; 45: 131–140.
- Bulut Y, Baysal Z. Removal of Pb(II) from wastewater using wheat bran. J Environ Manage. 2006; 78: 107–113.
- Gonzalez RG, Córdova FJC, Leon AMG, Regalado ES, Guzman NED, Rabago JJS. Lead biosorption onto coffee grounds: Comparative analysis of several optimization techniques using equilibrium adsorption models and ANN. J Taiwan Inst of Chem Eng. 2016; 00:1-10. Ho YS, Huang CT, Huang HW. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002; 37: 1421–1430.
- Ho YS, Ofomaja A E. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005; 40: 3455–3461.
- Saeed A, Iqbal M, Akhtar M W. Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater. 2005; 117: 65–73.
- El-Ashtoukhy, ESZ, Amin NK, Abdelwahab, O. Removal of lead(II) and copper(II) from aqueous solution using pomegranate peel as a new adsorbent. Desal. 2008; 223: 162–173.
- Randall JM, Hautala E, Donald G Mc. Binding of heavy metal ions by formaldehyde-polymerized peanut skins. J Appl Polymer Sci. 1978; 22: 379–387.
- Ucun H, Bayhan YK, Kaya K, Cakici A, Algur OF. Biosorption of lead(II) from aqueous solution by cone biomass of Pinus sylvestris. Desal. 2003; 154: 233–238.
- Ayyappan R, Sophia AC, Swaminathan K, Sandhya S. Removal of Pb(II) from aqueous solution using carbon derived from agricultural wastes. Process Biochemistry. 2005; 40: 1293–1299.
- Ronda A, Martine-Lara MA, Dionisio E, Blazquez G, Calero M. Effect of lead in biosorption of cupper in almond shell. J Tiwan Inst of Chem Eng. 2013; 44: 466-473.
- Balzquez G, Calero M, Ronda A, Tenorio G, Martine-Lara MA. Study of kinetics in biosorption of lead onto native and chemically treated olive stone. J Indust Eng chem. 2013; 20: 2754–2760.
- Olivares JC, Alonso C P, Díaz C B, Nuñez F U, Mercado MCC, Bilyeu B. Modeling of lead(II) biosorption by residue of allspice in a fixed column. J of Chem Eng. 2013; 228: 21-27.
- Hafshejani LD, Nasab SB, Gholami RM, Moradzadeh M, Izadpanah Z, Hafshejani SB. Removal of zinc and lead from aqueous solution by nanostructured cedar leaf ash as biosorbent. J Mol Liq.2015; 211: 448–456.
- Coelho GF, Gonçalves Jr AC, Tarley CRT, Casarin J, Nacke H, Francziskowski MA. Removal of metal ions Cd(II), Pb(II), and Cr (III) from water by the cashew nut shell Anacardium occidentale L. Ecol Eng J. 2014; 73: 514–525.
- Tasar S, Kaya F, Ozer A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng. 2014; 2: 1018–1026.
- Calero M, Pérez A, Blázquez G, Ronda A, Martín-Lara MA. Characterization of chemically modi?ed biosorbents from olive tree pruning for the biosorption of lead. Ecolo Eng. 2013; 58: 344-354.
- Martín-Lara MA, Blázquez G, Calero M, Almendros AI, Ronda A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fixed bed columns. I J of Mineral Proce. 2016; 148: 72–82.
- Morosanu I, Teodosiu, C, Paduraru C, Ibanescu D, Tofan L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. New biotechnol. 2016.
- Tyagi AK, Malik A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapors phase against food spoilage microorganisms. Food Chem. 2011; 126: 228–235.
- Khaled Si, Fawzi S, Wahid H, Khouja ML, Sadok B. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leave. Biol Res. 2015; 48: 7.
- Sarin V, Pant KK. Removal of chromium from industrial waste by using eucalyptus bark. Bioresour Technol.2006; 97: 15–20.
- Ghodbane I, Hamdaoui O. Removal of mercury(II) from aqueous media using Eucalyptus bark: kinetic and equilibrium studies. J Hazard Mater. 2008; 160: 310–309.
- Montgomery DC. Design and Analysis of Experiments. 5th edn. New York: John Wiley and Sons. 2001.
- Özer A, Gürbüz G, C alimli A, Körbahti BK. Biosorption of copper(II) ions on Enteromorpha prolifera: Application of response surface methodology (RSM). Chem Eng J. 2009; 146: 377–387.
- Langmuir I. The constitution and fundamental properties of solids and liquids. Part I Solids. J Amer Chemi Soc. 1916; 38: 2221–2295.
- Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906; 57: 385–470.
- Aharoni C, Ungarish M. Kinetics of activated chemisorption-part-2? Theoretical models. J Chem Soc Faraday Trans1. 1977; 73: 456-464.
- Dubinin MM, Zaverina ED, Radushkevich LV. Sorption and structure of active carbons. Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii.1947; 21: 1351–1362.
- Polanyi M. Theories of the adsorption of gases. A general survey and some additional remarks. Trans Faraday Soc. 1932; 28: 316–333.
- Naiya TK, Bhattacharya AK, Mandal SN, Das SK. The sorption of lead(II) ions on rice husk ash. J Hazard Mater. 2009; 163: 1254–1264.
- Lagergren S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar. 1898; 2: 1–39.
- Liang S, Guo X, Feng N, Tian Q. Adsorption of Cu(II) and Cd(II) from aqueous solution by mercapto acetic acid modified orange peel. Colloid and Surf. 2009; 73: 10–14.
- Bhattacharyya KG, Gupta SS. Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interface Sci. 2011; 162: 39–58.
- Weber WJ, Carrell Morris J. Kinetics of adsorption on carbon from solution. J Sanit Eng Div.1963; 89: 31–60.
- Shanthakumar S, Vilvanathan S. Biosorption of Co(II) ions from aqueous solution using Chrysanthemum indicum: Kinetics, equilibrium and thermodynamics. Proce Safe Environ Protect. 2015; 96: 98–110.
- Qaiser S, Saleemi AR, Umar M. Biosorption of lead from aqueous solution by Ficus religiosa leaves: batch and column study. J Hazard Mater. 2009; 166: 998– 1005.
- Chakravarty S, Ashok M, Sudha TN, Upadhyay AK, Konar J, Sircar JK, et al. Removal of Pb(II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J Hazard Mater.2010; 173: 502– 509.
- Chen H, Zhao J, Dai G, Wu J, Yan H. Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desal. 2010; 262: 174–182.
- Bingol D, Hercan M, Elevli S, Kilic E. Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresour Technol. 2012; 112: 111–115.
- Singh KK, Talat M, Hasan SH. Removal of lead from aqueous solutions by agricultural waste maize bran. Bioresour Technol. 2006; 97: 2124–2130.
- Suguna M, Narayana Reddy MV, Sreenivasulu V, Krishnaiah A. Modified leaf biomass for Pb(II) removal from aqueous solution: Application of response surface methodology. Ecol Eng. 2015; 83: 218–226.
- Özcana AS, Tunali S, Tamer A, Özcana A. Biosorption of lead(II) ions onto waste biomass of Phaseolus vulgaris L.: estimation of the equilibrium, kinetic and thermodynamic parameters. Desal. 2009; 244: 188–198.
- Tabaraki R, Nateghi A, Salman AA. Biosorption of lead(II) ions on Sargassum ilicifolium: Application of response surface methodology. I Biodeteri &Biodegra. 2014; 19: 145-152.