Research Article
Austin Chromatogr. 2014;1(1): 4.
A Validated Reversed-Phase HPLC Method for the Determination of Atorvastatin Calcium in Tablets
Simionato LD, Ferello L, Stamer SG, Repetto MF, Zubata PD and Segall AI*
Facultad de Farmaciay Bioquímica, Universidad de Buenos Aires, Argentina
*Corresponding author: Segall AI, Cátedra de Calidad de Medicamentos, Facultad de Farmaciay Bioquímica, Universidad de Buenos Aires, CONICET, Junín 956, 1113 Buenos Aires, Argentina
Received: August 25, 2014; Accepted: September 12, 2014; Published: September 17, 2014
Abstract
A Reversed-Phase Liquid Chromatographic (RP-LC) assay method was developed for the quantitative determination of atorvastatin calcium in the presence of its degradation products. The assay involved an isocratic elution of atorvastatin calcium in a LiChroCARTR 250*4 mm HPLC Cartridge LiChrospherR 100 RP-18 (5 μm) column using a mobile phase consisting of 0.1% acetic acid solution: acetonitrile (45:55, v/v), pH = 3.8. The flow rate was 0.8 mL/min and the analytes monitored at 246 nm. The assay method was found to be linear from 8.13 to 23.77 μg/mL. All the validation parameters were within the acceptance range. The developed method was successfully applied to estimate the amount of atorvastatin calcium in tablets.
Keywords: Atorvastatin calcium; RP-HPLC; Tablets assay
Introduction
Atorvastatin, 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-, calcium salt (2:1), trihydrate [R-(R*,R*)]-; (Figure 1) is a statin group medicine that reduces the level of serum cholesterol; thus it is used to treat hypercholesterolemia. Independent of the cholesterol-lowering property of statins they also have anti-inflammatory and immunomodulating effects. It is rapidly absorbed from the gastro-intestinal tract. It has low absolute bioavailability of about 12% due to presystemic clearance in the gastro-intestinal mucosa and/or first pass metabolism in the liver, its primary site of action. Atorvastatin is metabolized by cytochrome P450 3A4 to a number of compounds which are also active inhibitors of HMG-CoA reeducates. The mean plasma elimination half-life of inhibitory activity for HMG-CoA reeducates is approximately 20 to 30 hours due to the contribution of the active metabolites. It is 98% bound to plasma proteins. Atorvastatin is excreted as metabolites, primarily in the bile [1].
Figure 1: Atorvastatin Calcium.
Several HPLC methods were reported in the literature for the quantitative determination of atorvastatin calcium in biological samples alone [2-4], and with another active drug substance [5- 9]. Most of the analytical techniques for atorvastatin calcium described in the literature are based on the liquid chromatographic determination of this drug alone in pharmaceutical formulations [10-13] with another active drug substance [14-18]. Other analytical techniques such as spectroscopy [19-23], MALDI Mass spectrometry imaging [24] electrochemical [25] and capillary electrophoresis [26] has also been described. A reversed phase LC with UV detection for the quantitation of atorvastatin calcium in bulk material is described in United States Pharmacopeia [27].
The purpose of this work was to develop a procedure for the quantitation of atorvastatin calcium and its separation, mainly, from its related substances. In addition, forced degradation studies of atorvastatin calcium were performed to provide an indication of the specificity of the method. The method was also applied to four commercial formulations of the Argentinean market. The method was validated following the analytical performance parameters suggested by International Conference on Harmonization (ICH) [28].
Materials and Methods
Atorvastatin calcium trihidrate (98.8% calculated with reference to the dried substance) was purchased in Saporiti, Argentina. Acetonitrile and Methanol used were HPLC grade, Sintorgan (Buenos Aires, Argentina). Acetic acid was AR grade Sintorgan (Buenos Aires, Argentina). Distilled water was passed through a 0.45 μm membrane filter.
Equipment
The HPLC system consisted of a dual piston reciprocating Spectra Physics pump (Irvine, CA, United States, Model ISO Chrom. LC pump), a UV-Vis Hewlett Packard detector (Model 1050), a Hewlett Packard integrator (Loveland, CO, United States, Series 3395) and a Rheodyne injector (Model 7125).
Chromatographic conditions
The experiment was performed on a LiChroCARTR 250*4 mm HPLC Cartridge LiChrospherR 100 RP-18 (5 μm) Merck (Darmstadt, Germany). The separation was carried out under isocratic elution with 0.1% acetic acid solution: acetonitrile (45:55, v/v), pH = 3.8. The flow rate was 0.8 mL/min. The wavelength was monitored at 246 nm, and the injection volume was 20 μl. The HPLC was operated at ambient temperature. Under these conditions, the retention time (tR) of atorvastatin calcium was approximately 6.3 min.
Preparation of standard solution
An accurately weighed quantity of 25 mg of atorvastatin calcium was placed into a 100 mL volumetric flask, dissolved in 5 mL of methanol and taken to volume with mobile phase. Then, 4 mL were withdrawn in a 100 mL volumetric flask. The volume was made with mobile phase (Conc 20 μg/mL). The solutions were passed through a 0.45 μm nylon membrane filter before injection (25 mm disposable filter; Cat. N° R04SP02500 Osmonics Inc., Minnesota, USA).
Sample preparation
Approximately 25 mg of atorvastatin calcium raw material was placed into a 100 mL volumetric flask, dissolved in 5 mL of methanol and taken to volume with mobile phase. Then, 4 mL were withdrawn in a 100 mL volumetric flask. The volume was made with mobile phase (Conc 20 μg/mL). The solutions were passed through a 0.45 μm nylon membrane filter before injection (25 mm disposable filter; Cat. N° R04SP02500 Osmonics Inc., Minnesota, USA).
Preparation of comercial formulations
Twenty tablets were weighed and finely powered and an accurately weighed powder sample equivalent to one tablet was transferred to a 50 ml volumetric flask; 10 ml of methanol was added and the flask was kept in an ultrasonic bath during 5 min. The mixture was then diluted to 50 ml with mobile phase. 1 mL was withdraw in a 10 mL volumetric flask and diluted to volume with mobile phase. The solutions were passed through a 0.45 μm nylon membrane filter before injection (25 mm disposable filter; Cat. N° R04SP02500 Osmonics Inc., Minnesota USA).
Method validation
System suitability: The Relative Standard Deviations (RSD) values of the peak area, tailing factor, retention time, capacity and theoretical plates were the chromatographic parameters selected for the system suitability test [27].
Specificity: Forced degradation studies were performed to evaluate the specificity of the method. Degraded samples were prepared by refluxing 1.25 mg/mL atorvastatin calcium working standard with acid (1N hydrochloric acid), base (1N NaOH), water, 30% hydrogen peroxide and refluxing for 1 hour. The drug was subjected to thermal degradation in solution state in a closed container in an oven at 80°C for 2 h and to photochemical degradation, (a solution was transferred to a container and exposed to daylight for 96 h). After each degradation treatment, samples were allowed to cool at room temperature and diluted, to the same concentration as that of the standard solution, after being neutralized. Further, samples were analyzed using the methodology and the chromatographic conditions described.
Linearity: The linearity solutions were prepared at five concentrations levels from 40 % (w/v) to 125 % (w/v) of analytes concentration. Triplicate injections of 20 μL were made and chromatograph under the conditions described above. The drug was evaluated and peak areas were recorded. A calibration curve was plotted by taking the peak area on y-axis and respective concentration of drug on x-axis. The calibration curve was constructed and evaluated by its coefficient of determination (r2) and by least-squares linear regression analysis.
Precision and accuracy: Six replicated of standard solution were analyzed to assess system precision. Both reproducibility and accuracy studies were evaluated by carrying out nine independent assays at concentration levels of 80, 100 and 120 % (w/v) (3 samples each) of a commercial formulation of atorvastatin calcium. The amount of atorvastatin calcium recovered was calculated.
Robustness: The robustness was performed by deliberately changing the chromatographic conditions. The relative organic portion ratio of the eluent was varied by 45 to 55 %, while pH was adjusted to 2.8 and 4.3. The RSD, retention time, tailing, and theoretical plates were evaluated.
Results and Discussion
The described reverse-phase liquid chromatography method was developed to provide a rapid quality control determination of atorvastatin calcium in tablets. Validation of the method was performed according to ICH. This method uses a simple mobile phase. All samples were analyzed using the assay chromatographic conditions described.
The analytical column was equilibrated with the eluting solvent system used. After an acceptably stable baseline was achieved, the standards and then the samples were analyzed.
The system suitability results were calculated according to the USP 35 <621> (27) from typical chromatograms. The system precision is a measure of the method variability that can be expected for a given analyst performing the analysis and was determined by performing six replicate analyses of the same working solution. The Relative Standard Deviation (RSD) obtained was 0.6%. Peak asymmetry or tailing factor, T, was calculated as T=W0.05/2f; where W0.05 is the distance from the leading edge to the tailing edge of the peak, measured at 5% of the peak height from the baseline and f is the distance from the peak maximum to the leading edge of the peak. The tailing factor did not exceed 1.5 The RSD of peak area response and retention time showed the satisfactory repeatability of the system (< 1.5%) (Table 1).
Parameter
Minimun value
Maximun value
Average
RSD (%)
Retention time
6.292
6.331
6.312
0.44
Area
6924058
7088160
7006109
1.66
Capacity
2.146
2.165
2.155
0.62
Asimetry factor
0.98
1.00
0.99
1.43
Theoretical plates
2534
2565
2550
0.86
Table 1: System Suitability.
Degradation was indicated in the stressed sample by a decrease in the expected concentration of the drug and increased levels of degradation products. Atorvastatin calcium was degraded to different products under acid, base, oxidation, hydrolysis and photolysis (Table 2). In addition, there was no interference regarding the retention time of atorvastatin calcium and its degradation products.
Condition
Time (h)
% of Atorvastatin
RRT of degradation products
Acid (1 N HCl, reflux)
1
18.5
1.34, 1.43, 2.14
Base (1 N NaOH, reflux)
1
79.3
0.39, 0.46, 0.57
Hydrogen peroxide 100 vol (reflux)
1
7.8
0.33, 0.43, 0.72, 0.78, 1.68, 2.90
Water (reflux)
1
85.9
0.38
Heat dry, 80°C (solution)
2
97.9
Non detected
Daylight exposure
96
56.6
0.77, 1.35, 1.64
Table 2: Selectivity.
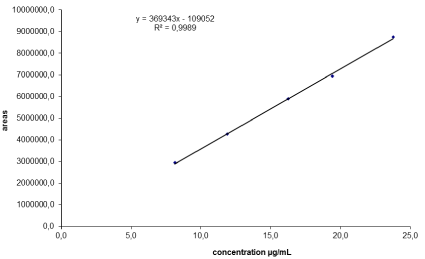
The linearity of the HPLC method was determined by analysis of three replicates of five concentrations of standard solutions (ranging from 8.13 and 23.77 μg/mL). The calibration curve showed good linearity over the concentration range. The correlation coefficient (“r”) value was 0.9994. Typically, the regression equation for the calibration curve was found to be y = 369342.9x – 109051.8. The linearity of the calibration graphs was validated by the high value of the correlation coefficient and the intercept value that was not statistically (p = 0.05) different from zero (Figure 2 and Table 3).
Figure 2: Linearity of the method.
% of nominal value
Concentration (μg/mL)
Average peak area response
RSD
%
40
8.13
2938581
0.6
60
11.88
4266208
1.2
80
16.26
5889101
0.9
100
19.42
6949213
0.3
125
23.77
8759624
0.1
Slopea
369342.9 ± 929563,0
Interceptb
-109051.8 ± 15630862,6
Table 3: Linearity.
The precision is usually expressed as the RSD of a series of measurements. The reproducibility and accuracy studies were evaluated by recovery studies with 9 samples of one commercial formulation studied (n = 3 for 80%, 100% and 120%) indicated that the mean recovery was 99.4 %, and the RSD was 0.63%.
Method accuracy was also demonstrated by plotting the amount of atorvastatin calcium found against the amount present in the sample, both expressed in mg. Linear regression analysis rendered slopes not significantly different from 1 (t test p=0.05), intercepts not significantly different from zero (t test p=0.05), and r = 0.9992. The experimental t of the recovery percentage was also studied, showing a value of 2.00, below the 2.306 established in the tabulated t (95% level of probability, 8d.f) (Table 4).
% of nominal value
Added Amount
(mg))
Found amount
(mg)
Recovery
(%)
Average recovery
(n=3)
RSD
(%)
80
20.2
20.8
21.0
20.1
20.5
20.7
99.5
98.8
98.6
99.0
0.48
100
26,2
25.2
24.7
26.1
24.8
24.7
99.7
98.5
100.0
99.4
0.80
120
31.1
30.2
29.7
31.4
30.2
29.5
100.1
100.0
99.5
99.9
0.32
Mean (n=9)
99.4
0.63
Table 4: Precision and Accuracy.
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters, and provides an indication of its reliability during normal usage.
Robustness of the method was investigated under a variety of conditions including changes of pH and percentage of acetonitrile in the mobile phase.
The effect on retention time, theoretical plates and tailing factor can be seen in Table 5. A decrease in acetonitrile proportion increases both retention time and theoretical plates. It was found that retention time of atorvastatin calcium ranges from 0.3 cm/min by pH changes.
Mobile Phase
RT
Tailing factor
N
0.1% Acetic Acid solution –Acetonitrile (45:55, v/v)
pH: 3.8
6.30
1.11
2545
0.1% Acetic Acid solution - Acetonitrile (45:55, v/v)
pH: 2.8
6.57
1.00
2767
0.1% Acetic Acid solution - Acetonitrile (45:55, v/v)
pH: 4.3
6.01
1.00
2312
0.1% Acetic Acid solution - Acetonitrile (55:45, v/v)
15.24
1.33
14865
0.1% Acetic Acid solution - Acetonitrile (50:50, v/v)
9.16
1.00
5365
Table 5: Robustness.
The results of the evaluation of the four market products can be in Table 6.
Brand
Average %
RSD%
A
92.1
1.11
B
105.0
0.53
C
92.9
1.54
D
98.6
0.82
Table 6: Commercial formulations assay.
Conclusion
A straightforward, specific, linear, precise and accurate RP-HPLC method has been developed and validated for quantitative determination of atorvastatin calcium in tablets. The method is very simple and specific, as the peak is well separated from its impurities, which makes it especially suitable for routine quality control analysis work.
Acknowledgement
This work was supported by a grant from UBA (No. 20020100100816) and from CONICET, PIP N°: 11420110100380 (both to A. I. S.).
References
- Martindale. The Complete Drug reference. 32th edn. Pharmaceutical Press, London. 1999; 1268.
- Fernández-Varón E, Bermejo R, Ayala I, García-Pérez B, Tvarijonaviciütè A, Cárceles C. Desarrollo y validación de una técnica de cromatografía líquida de alta resolución para la determinación de atorvastatina en plasma en un biomodelo experimental de arteriosclerosis en pollo. Clin Invest. Arterioscl. 2005; 17: 223-227.
- Chou YC, Wang YK, Charng MJ, Ueng YF. Determination of serum atorvastatin concentrations in lipid controlling patients with and without myalgia syndrome. J Food Drug Anal. 2013; 21: 147-153.
- Partani P, Manaswita Verma S, Gurule S, Khuroo A, Monit T. Simultaneous quantitation of atorvastatin and its two active metabolites in human plasma by liquid chromatography/(-) electrospray tandem mass spectrometry. J Pharm Anal. 2014; 4: 26-36.
- Shah Y, Iqbal Z, Ahmad L, Khan A, Khan M. I, Nazir S, et al. Simultaneous determination of rosuvastatin and atorvastatin in human serum using RP-HPLC/UV detection: Method development, validation and optimization of various experimental parameters. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879: 557-563.
- Nováková L, Šatínský D. Solich P. HPLC methods for the determination of simvastatin and atorvastatin. Trends Anal. Chem, 2008; 27: 352-367.
- Zhou Y, Li J, He X, Jia M, Liu M, Li H, et al. Development and validation of a liquid chromatography-tandem mass spectrometry method for simultaneous determination of amlodipine, atorvastatin and its metabolites ortho-hydroxy atorvastatin and para-hydroxy atorvastatin in human plasma ans its application in a bioequivalence studies. J Pharm Biomed Anal. 2013; 83: 101-107.
- Abdelbary G, Nebsen M. Application of a novel UPLC-MS/MS method for the pharmacokinetic/bioequivalence determination of atorvastatin and ezetimibe in human plasma. J Pharm Res. 2013; 7: 24-32.
- Polagani SR, Pilli NR, Gajula R, Gandu V. Simultaneous determination of atorvastatim, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. J Pharm Anal. 2013; 3: 9-19.
- Ertürk S, Sevinç Aktas E, Ersoy L, Fiçicioglu S. An HPLC method for the determination of atorvastatin and its impurities in bulk drug and tablets. J Pharm Biomed Anal. 2003; 33: 1017-1023.
- Gupta LK. Spectroscopic characterization and quantitative determination of atorvastatin calcium impurities by novel HPLC method. Spectrochim Acta A Mol Biomol Spectrosc. 2012; 97: 495-501.
- Sharaf El_Din MMK, Salama FMM, Nassar MWI, Attia KAM, Kaddah MMY. Validated spectrofluorimetric method for the determination of atorvastatin in pharmaceutical preparations. J Pharm Anal. 2012; 2: 200-2015.
- Zaheer Z, Farooqui MN, Mangle AA, Nikalje AG. Stability-indicating high performance liquid chromatographic determination of atorvastatin calcium in pharmaceutical dosage form. Afr J Pharm Pharmacol. 2008; 2: 204-210.
- Sangshetti JN, Aqeel M, Zaheer Z, Ahmed RZ, Dehghan MHG, Gonjari I. Development and validation of RP-HPLC method for determination of atorvastatin calcium and nicotinic acid in combined tablet dosage form. J Saudi Chem Soc. 2012.
- Mohammadi A, Rezanour N, Ansari Dogaheh M, Ghorbani Bidkorbeh F, Hashem M, Walker RB. A stability indicating high performance liquid chromatographic (HPLC) assay for the simultaneous determination of atorvastatin and amlodipine in commercial tablets. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 846: 215-221.
- Janardhanan VS, Manavalan R, Valliappan K. Chemometric technique for the optimization of chromatographic system; Simultaneous HPLC determination of rosuvastatin, telmisartan, ezetimibe and atorvastatin used in combined cardiovascular therapy. Arab J Chem. 2012.
- Kumar Talluri MVN, Kayankar A, Ragampeta S. Synchronized separation of atorvastatin-an antihyperlipidemic drug with antihypertensive, antidiabetic, antithrombotic drugs by RP-LC for determination in combined formulations. J Pharm Anal. 2012; 2: 285-292.
- Rajavel IR, Ganesh M, Jagadeeswaran M, Srinivasan K, Valarmathi J, Sivakumar T. RP-HPLC method for the simultaneous determination of aspirin, atorvastatin and pioglitazone in capsule dosage form. Asian J Research Chem. 2008; 1: 40-42.
- Dinakaran SK, Alluri B, Annareddy KR, Ayyagari VS, Avasarala H, Kakaraparthy R, et al. Spectrophotometric method development and validation for atorvastatin calcium and nifedipine HCl in bulk and tablet dosge form using absorption ratio method assay of atorvastatin and nifedipine. J Pharm Res. 2013; 7: 666-669.
- Issa MM, Nejem RM, Shanab AA, Hegazy ND, van Staden RIS. Comparative study of three modified numerical spectrophotometric methods: An application on pharmaceutical ternary mixture of aspirin, atorvastatin and clopedrogrel. Spectrochim Acta A. 2014; 128: 514-521.
- Baghdady YZ, Al-Ghobashy MA, Abdel-Aleem AAE, Weshahy SA. Spectrophotometricand TLC-densitometric methods for the simultaneous determination of ezetimibe and atorvastatin calcium. J Adv Res. 2013; 4: 51-59.
- Belal TS, Daabees HG, Abdel-Khalek MM, Mahrous MS, Khamis MM. New simple spectro metricmethod for determination of the binary mixtures (atorvastatin calcium and ezetimibe; candesartan cilexetil and hydrochlorothiazide) in tablets. J Pharm Anal. 2013; 3: 118-126.
- Ilango K, Kumar PS. Validated spectrophotometric methods for the simultaneous determination of telmisartan and atorvastatin in bulk and tablets. Pharm Methods. 2012; 3: 112-116.
- Rodrigues LR, de Oliveira DN, Ferreira MS, Catharino RR. In situ assessment of atorvastatin impurity using MALDI mass spectrometry imaging (MALDI-MSI). Anal Chim Acta. 2014; 818: 32-38.
- Kamalzadeh Z, Shahrokhian S. Electrochemical determination of atorvastatin on nano-scaled polypyrrole film. Bioelectrochemistry. 2014; 98: 1-10.
- Alshehri MM. A validated capillary electrophoresis method for simultaneous determination of ezetimibe and atorvastatin in pharmaceutical formulations. Saudi Pharm J. 2012; 20: 143-148.
- The United States Pharmacopeia. 35th edn. US. Pharmacopeial Convention, Rockville. 2013.
- International Conference on Harmonization. ICH Q2 (R1) Guideline on Validation of Analytical Procedures: Text and Methodology. 2005.