
Research Article
Chronic Dis Int. 2014;1(2): 5.
Red Blood Cell Size Factor (Rsf) Potential Marker of Iron Restricted Erythropoiesis in Hemodialysed Patients
Eloisa Urrechaga1*, Ramón Saracho2, Luís Borque3 and Jesus F Escanero3
1Hematology Laboratory, Hospital Galdakao – Usansolo Galdakao, Spain
2Department of Nephrology, Hospital Galdakao – Usansolo Galdakao, Spain
3Department of Pharmacology and Physiology, University of Zaragoza, Spain
*Corresponding author: Eloisa Urrechaga, Hematology Laboratory, Hospital Galdakao–Usansolo, 48960 Galdakao, Vizcaya, Spain
Received: December 08, 2014; Accepted: December 15, 2014; Published: December 16, 2014
Abstract
Background: Reticulocyte hemoglobin content (CHr) is incorporated to National Kidney Foundation (NKF-K/DOQI) guidelines for the monitoring of erythropoiesis-stimulating agents therapy in dialysed patients. The measurement of CHr has been restricted to the analyzers of a single manufacturer, Siemens. Red blood cell size factor (RSf) is a parameter provided by Beckman- Coulter which averages the volume of erythrocytes (MCV) and the volume of reticulocytes (MRV)
Aim: we aimed to investigate the reliability of RSf for assessing iron status in hemodialysed patients, in comparison with CHr.
Methods: A prospective study included 90 healthy individuals, 70 Iron Deficiency Anemia (IDA) and 55 dialysed (HD) patients; the hemograms were run in parallel in LH 750 (Beckman-Coulter) and Advia 2120 (Siemens) analyzers.
HD patients were included in a longitudinal study along one month of follow up.
Results: Good correlation between CHr and RSf was observed, r =0.85.
ROC curve analysis for RSf in the diagnosis of iron restricted erythropoiesis, defined by CHr < 29 pg.
AUC 0.952 (95 % CI 0.902-0.981) sensitivity 80.0 % (95 % CI 68.7- 88.6 %), specificity 92.5 % (95 % CI, 83.4- 97.5 %), cut off 92.2 fL. Along the four weeks follow up of the longitudinal study Hb, CHr and RSf remained constant, correlation between CHr and RSf r=0.744.
Conclusion: This study shows a good level of agreement between RSf and CHr. RSf could be a reliable parameter for the study of erythropoiesis in dialysed patients.
Keywords: Anemia; Haemodialysis; Iron monitoring; Red blood cell size factor; Reticulocyte haemoglobin content
Introduction
Anemia is one of the most characteristic manifestations of Chronic Renal Disease (CKD) and may be a product not only of decreased erythropoietin production, but also of decreased iron availability, both conditions lead to impaired erythropoiesis.
Recombinant human erythropoietin (rHuEpo) has been available for treatment of renal anemia since 1989 [1]. However, rHuEpo therapy results in functional iron deficiency, due to insufficient iron stores for the accelerated erythropoiesis [2].
Iron deficiency is the main cause of suboptimal response to erythropoietin in dialysis patients. Maintenance iron supplementation is required to successfully treat anemia. Long term orally administered iron therapy is limited by noncompliance, gastrointestinal side effects, insufficient absorption and drug interaction; intravenous iron compounds are used to treat dialysis patients who become iron deficient [3].
Monitoring erythropoietin treated patients’ iron status is important to detect iron deficiency and avoid the adverse effects of iron medication [4-6].
Biochemical indicators of iron metabolism (serum iron, transferrin saturation, serum ferritin) although widely used, may be influenced by the acute phase response, which complicates clinical interpretation of the test results. Serum ferritin, an indicator of iron storage but not of iron supply, is an acute phase reactant and its levels are affected by inflammation, serum ferritin levels might not reflect true iron stores.
Transferrin is a negative acute phase reactant, rendering the calculation of transferrin saturation unreliable in this case [7,8]. Transferrin fluctuates due to the diurnal variation of serum iron and is affected by nutritional status, leading to a lack of sensitivity and specificity in assessing iron’s availability [9]. For these reasons, an iron deficient erythropoietic response to rHuEpo may occur despite normal serum ferritin and transferrin values.
Interest has been generated in the use of erythrocyte and reticulocyte new indices available on the modern analyzers based on flow cytometry technology.
Measurement of reticulocyte number and cellular characteristics may provide useful information about marrow erythropoietic activity [10]. The potential use of new reticulocyte parameters in the diagnosis of anemia and in monitoring the bone marrow erythropoietic activity has been assessed [11-13].
The measurement of reticulocyte hemoglobin content (CHr) is an assessment of the incorporation of iron into hemoglobin and thus an estimate of the recent functional availability of iron into the erythron [14,15].
CHr has been used as a diagnostic tool, together with biochemical markers, to distinguish Iron Deficiency Anemia (IDA) from Anemia of the Chronic Diseases (ACD), and is incorporated to National Kidney Foundation, Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) guidelines for the monitoring of recombinant human erythropoietin rHuEPO therapy [16-18].
The measurement of CHr has been restricted to the analyzers of a single manufacturer, Siemens (Siemens Medical Solutions Diagnostics, Tarrytown NY, USA).
Beckman-Coulter (Beckman Coulter Inc., Miami Fl, and USA) has proposed a new parameter Red blood cell Size Factor, RSf
RSf averages the volume of mature red cells (MCV) and the volume of reticulocytes (MRV), both related to erythropoietic activity and hemoglobinization.
When MCV is measured what is measured is the average size or volume of the red blood cells produced in a period of 120 days before the sample is drawn; on the other hand, when MRV is measured what is measured is the size of the recently produced red cells, within a period of less than 2 days: the measurement of reticulocyte number and volume provides real time data regarding certain aspects of erythropoiesis, such as iron availability, that can influence the dimensions of red cells
Both in mature red cells and reticulocytes above 90 % cellular contents are Hb the size of these cells directly correlates with the Hb content of the cells. RSf, as a product function of MCV and MRV reflects indirectly the cellular Hb content of both cell populations; adding the square root averages both volumes and the units in which RSf is expressed are femtoliters.
Examining both precursors and mature red cells provides an opportunity to detect and monitor acute and chronic changes in cell volume related to cellular hemoglobin status and the amount of the iron supply.
RSf reference range and values in different clinical conditions have been reported and its value for the study of bone marrow erythropoietic activity, with the same clinical meaning as CHr, assessed [19].
The aim of this study was to evaluate the overall performance of the new parameter RSf in a cohort of dialysis patients in terms of concordance with CHr as well as the potential clinical utility in monitoring functional iron deficiency in those patients.
Material and Methods
Patients selection
Healthy group: 45 male and 45 female healthy adult subjects, with no clinical symptoms of disease and results within reference ranges in the complete blood count and biochemical iron metabolism markers, were recruited.
Iron deficiency group: IDA patients fulfilled traditional diagnostic criteria for Iron deficiency anemia diagnosis, Serum Iron < 7.5 μmol/L, Transferrin saturation <20%, Ferritin <50 μg/L, and Hemoglobin (Hb) <12.0 g/dL (females) or < 13.0 g/dL (males), were included before iron treatment.
Hemodialysed group (HD): 55 patients (33 male, 22 female, mean age 61±8 years). All of them were being treated with intermittent in center hemodialysis (standard 4 h bicarbonate dialysis three times per week) and were treated with rHuEPO for at least 3 months and were in the maintenance phase of their treatment with stable rHuEPO doses (80-120 units/Kg/) for at least 4 weeks, in order to meet the recommendations of the European Best Practice Guidelines target hemoglobin concentration of 11.0 g/dL [20]. All were given regular iron therapy (intravenous iron gluconate 30 to 60 mg/week), to maintain the iron availability and transferrin saturation 20 %, and were receiving folate (5 mg orally twice a week) and vitamin B12 (1000 μg three times a week) supplementation.
None of the patients had received blood transfusions nor were affected of bleeding episodes in at least the previous month before starting the study.
A prospective study was conducted including the three groups of patients healthy, IDA and HD (baseline data).
HD patients were included in a longitudinal study and followed during a four weeks period. Blood samples were drawn before the hemodialysis session corresponding to the first day of the dialysis week.
Four samples of each patient had been analysed, at the end of the longitudinal study 220 samples have been processed.
The dose of rHuEPO remained unchanged during the whole period under study.
The protocol was approved by the Ethics Committee of Galdakao - Usansolo Hospital.
Laboratory assays
Blood samples were collected in K2EDTA anti-coagulant tubes (VacutainerTM Becton-Dickinson, Rutherford NJ, USA), were run sequentially on both the LH 750 (Beckman Coulter Inc., Miami Fl, USA) and ADVIA 2120 (Siemens Medical Solutions Diagnostics, Tarrytown NY, USA), within 6 h of collection.
Serum Iron, ferritin and transferrin were measured using a Cobas c 711 chemistry analyzer (Roche Diagnostics, Mannheim, Germany) and reactive from Roche Diagnostics, (Mannheim, Germany).
Serum iron was measured using an assay based on a FerroZine Submit your Manuscript | www.austinpublishinggroup.com reaction (Iron Gen2). Serum ferritin was measured using an immunoturbidimetric assay (Tina-quant Ferritin Gen3). Transferrin was measured was measured using an immunoturbidimetric assay (Tina-quant TF2).
Statistical analysis
Statistical software package SPSS (SPSS; Chicago, IL 60606, USA) version 19.0 for windows was applied for statistical analysis.
Kolmogorov-Smirnoff test was applied to verify the normal distribution of values of the tests under investigation.
Correlation coefficients were calculated by Pearson method.
Independent samples t test was performed in order to detect statistical deviations between the groups of patients; p values less than 0.05 were considered to be statistically significant.
Receiver Operating Characteristic (ROC) curve analysis was utilized to illustrate the diagnostic performance of RSf in the assessment of erythropoietic function. Iron restricted erythropoiesis was defined by CHr < 29 pg.
For the longitudinal study changes in the variables were examined by means of a linear mixed model.
Results
The biochemical and hematological data of the patients are summarised on Table 1; data of the HD group are the values in the first week of the study (baseline).
RBC 1012/L
Hb
g/dL
MCV fL
MCH pg
MCHC g/dL
Ret %
RSf fL
CHr pg
Iron �mol/L
Transf g/L
Ferritin �g/L
Sat %
Healthy
mean(SD)
4.9
(0.27)
15.4
(0.64)
91.1
(2.55)
31.3
(1.53)
34.3
(0.52)
1.3
(0.48)
100.9
(5.3)
33.2
(1.6)
16.5
(0.62)
2.53
(21)
75
(12)
31
(1.9)
IDA mean
(SD)
4.6
(0.61)
9.5
(1.42)
70
(10.3)
22.5
(4.23)
32.0
(1.73)
2.1
(1.14)
88.1
(7.8)
24.5
(3.4)
4.8
(2.15)
3.28
(56)
18
(10)
6
(3.1)
HD mean
(SD)
3.8
(0.46)
11.4
(1.11)
94.9
(5.9)
30.2
(2.0)
31.8
(1.1)
1.7
(0.93)
103.5
(7.9)
31.6
(2.55)
11.5
(5.4)
1.63
(36)
404
(228)
28
(14)
SD: Standard Deviation; RBC: Red Blood Cells; Hb: Hemoglobin; MCV: Mean Cell Volume; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; Ret: % reticulocyte; RSf: Red blood cell Size factor; CHr: reticulocyte Hemoglobin Content; Transf: Transferrin; Sat: % transferrin Saturation.
Table 1: Baseline hematological and biochemical values in 90 healthy individuals, 70 Iron Deficiency Anemia (IDA) and 55 hemodialysed (HD) patients.
RSf results obtained in the healthy subjects group were within the reference range 91.1-116.1 fL.
The hematological and biochemical parameters of HD patients were statistically different (p<0.0001) from the values obtained in IDA patients.
Hb, Mean Cell Hemoglobin (MCH), Mean Cell Hemoglobin Concentration (MCHC), % reticulocytes and CHr values observed in the HD group showed an intermedia position between healthy subjects and IDA patients, reflecting the reduced iron availability for hemoglobin synthesis during the increased erythropoietic activity in response to hormone stimulation.
Mean Cell Volume (MCV) mean value was higher in HD group than in healthy subjects, because 9 patients included in the present study (16 %) were macrocytic, with MCV values over 98 fL.
CHr and RSf values in IDA patients were significantly lower than the values obtained in the healthy group (p<0.0001). CHr and RSf were significantly higher in HD and the healthy group than in IDA patients p<0.001.
Good correlation between CHr and RSf in the three groups was found r=0.85 (p<0.0001, 95 % CI 0.822 - 0.90) (Figure 1).
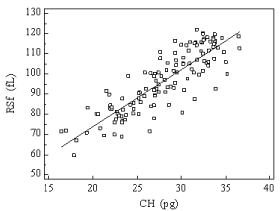
Figure 1: Relationship between reticulocyte hemoglobin content (CHr) and
Red blood cell Size factor (RSf) values in a group of 90 healthy individuals, 70
Iron Deficiency Anemia (IDA) and 55 hemodialysed (HD) patients. Correlation
coefficient calculated by Pearson method r = 0.85.
Diagnostic performance of RSf in the detection of iron restricted erythropoiesis defined by CHr < 29 pg gives the following data: AUC 0.952 (95 % CI 0.902-0.981) sensitivity 80.0 % (95 % CI 68.7- 88.6 %), specificity 92.5 % (95 % CI, 83.4 - 97.5 %), cut off 92.2 fL.
Along the four weeks follow up Hb, CHr and RSf remained constant (Table 2).
Weeks
1
2
3
4
Hb (g/dL)
11.4 (1.11)
11.1 (1.6)
p=0.25
10.8 (2.1)
p=0.40
11.2 (2.3)
p=0.34
CHr (pg)
31.6 (2.55)
32.1 (1.1)
p=0.81
31.8 (2.1)
p=35
32.3 (2.3)
p=0.23
RSf (fL)
103.5 (7.9)
106.8 (5.1)
p=0.06
104.8 (6.7)
p=0.08
102.5 (5.7)
p=0.08
CHr: reticulocyte Hemoglobin Content; RSf: Red blood cell Size factor.
Table 2: Baseline and evolution of hematological parameters, mean (standard deviation), in 55 hemodialysis patients along four weeks follow-up. p, significance level of the differences, the value of each column is compared with the value of the previous column.
Correlation between CHr and RSf in the HD patients in the follow up period r=0.74 (p<0.0001, 95 % CI 0.684-0.821).
Fifty four HD patients maintained erythropoiesis at a good rate during the period studied, as CHr values higher than 29 pg demonstrated. One patient had CHr values 27-28 pg along the follow up but the values obtained for RSf were within the reference range. Four patients (36 % of the macrocytic patients) presented RSf values higher than 116.1 fL while CHr ranged 34-37 pg.
Discussion
Anemia in chronic renal failure patients is multifactorial. The anemia is generally normocytic resulting from impaired red cell production due to defective erythropoietin production; other factors complicate the anemia: the retention of toxic substances inhibits erythropoiesis and shorts erythrocyte survival; the blood loss and reduced life span of red blood cell related to dialysis treatment; association with inflammatory processes; chronic mucosal blood loss due to uremic coagulopathy; poor iron absorption in the gastrointestinal tract iron or folate deficiency and aluminium toxicity [21].
Despite the prevalent use of rHuEPO therapy, anemia is a frequent finding in hemodialysis patients. The major cause of resistance to rHuEPO is iron deficiency. Absolute iron deficiency is easy to detect. However, functional iron deficiency, defined as a positive response to further iron supplementation in the absence of absolute iron deficiency, remains a daily challenge for nephrologists.
Iron deficiency should be corrected with a strict schedule in order to prevent iron accumulation associated complications, such as anaphylaxis, hemosiderosis, hepatic dysfunction, cardiovascular disease and infection [22].
The most important question regarding therapy in these patients is which are the best parameters to assess the iron available for erythropoiesis. New laboratory parameters are reported by different manufacturers as potential tools for anemia and iron restricted erythropoiesis diagnosis.
The diagnosis and monitoring of the response to erythropoietin therapy presumes the use of highly specific and sensitive tests, which can be useful to prevent iron overload and the negative side effects associated to it.
The blood concentration of reticulocytes represents a quantitative measure of erythropoietic activity, while the reticulocyte parameters provide real-time information about the quality of erythropoiesis (iron deficient or adequate iron supply).
Several authors have studied the value of CHr as an indicator of iron deficiency in dialysis patients and different cut off values have been chosen [23-26]. As CHr is a parameter available only in one manufacture’s analyzers the question is if there is any other parameter with the same clinical meaning of CHr.
In the initial phases of functional iron deficiency and restricted erythropoiesis, fluctuations in the iron supply to the bone marrow yield decreased Hb production in reticulocytes, resulting in low CHr and also low MRV [13]. The same but reversed can be assumed when the iron therapy has been applied and bone marrow activity is recovering.
A good correlation between RSf and CHr has been stated. In the prospective study both parameters showed the same trend in the three groups.
CHr and RSf values in IDA patients were significantly lower than the values obtained in the healthy group (p<0.0001), results compatible with impaired hemoglobinization due to iron efficiency. CHr and RSf were significantly higher in HD and the healthy group than in IDA patients (p<0.001) reflecting the normal hemoglobinization due to treatment.
The diagnostic performance of RSf in the detection of iron restricted erythropoiesis, defined as CHr < 29 pg was good; a threshold value of 92.2 fL provides sensitivity 80.0 % and specificity 92.5 %.
The present study demonstrates the correspondence between RSf and CHr in HD patients. This correspondence was independent from clinical changes and maintained along the follow up.
Although the values of both parameters ran parallel during the follow up period, showing that the patients were in a stable phase of the disease, some discrepancies arouse and Pearson coefficient was lower than the value obtained in the prospective study.
Only one patient presented CHr < 29 pg during the period under study. This patient had a slight macrocytosis and RSf values were 96-99 fL, within the reference range. Sixteen percent of the dialysis patients included in the present study showed macrocytosis, with MCV over 98 fL and, during the follow up, 4 of them (36 %) had RSf values higher than the upper limit of the reference range 116 fL.
Some hemodialysed patients present MCV and MRV values higher than reference range, independently of rHuEPO therapy and unrelated to folate and vitamin B12 deficiency. Speculation on the pathogenesis of this macrocytosis mainly incriminates the amount of urea and other products as cystathionine, which interfere with the folate cycle and eventually leads to DNA and RNA synthesis impairment [27,28].
RSf joins erithrocyte and reticulocyte volumes; it must be assumed that when macrocytosis is present the RSf values could be higher than the reference range, but may be not directly correlated with the Hb content of the macrocytic red cells and a good level of erythropoiesis status.
But it must be taken into account that CHr is not independent from the cell volume. CHr value is the product of hemoglobin concentration and the cell volume [13]. Discrepancies in cell size, Hb concentration and Hb content in some hemodialysis patients have been reported [29].
High CHr value accompanied with high percentage of hypochromic and macrocytic erythrocytes are found in some hemodialysis patients. These analitycal results may be due to the accelerated erythropoiesis, effect of rHuEPO therapy and the rapid increase in the number of erythrocytes; the younger erithroid population released from bone marrow are characterised by their greater volume with a stable Hb content but a decreased concentration [27].
In summary, the present study shows a good level of agreement between RSf and CHr: a high correlation between these parameters has been shown.
It has been stated that RSf can be a suitable parameter for the study of bone marrow erythropoietic activity in dialysed patients, but it must be take into account the presence of macrocytosis to correctly assess the results.
Although further investigations are needed in order to verify these results in dialysed patients in different clinical situations, the conclusion is that RSf seems to be an acceptable alternative to CHr for Beckman-Coulter costumers in the routine practice.
RSf could be useful in assessing functional iron deficiency and therefore improve anemia management in patients receiving hemodialysis, and in conjunction with standard parameters, could enable the diagnosis to be made rapid and accurately.
References
- Eschbach JW, Downing MR, Egrie JC, Browne JK, Adamson JW. USA multicenter clinical trial with recombinant human erythropoietin (Amgen). Results in hemodialysis patients. Contrib Nephrol. 1989; 76: 160-165.
- Cavil I, Macdougall IC. Erythropoiesis and iron supply in patients treated with Erythropoietin. Erythropoiesis. 1992; 3: 50-55.
- Macdougall IC. Poor response to erythropoietin: practical guidelines on investigation and management. Nephrol Dial Transplant. 1995; 10: 607-614.
- Sunder-Plassmann G, Horl WH. Laboratory diagnosis of anaemia in dialysis patients: use of common laboratory tests. Curr Opin Nephrol Hypertens. 1997; 6: 566-569.
- Kletzmayr J, Sunder-Plassmann G, Horl WH. High dose intravenous iron: a note of caution. Nephrol Dial Transplant. 2002; 17: 962-965.
- Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002; 40: 90-103.
- Mast A. The clinical utility of peripheral blood tests in the diagnosis of iron deficiency anemia. Bloodline. 2001; 1: 7-9.
- Coyne D. Iron indices: what do they really mean? Kidney Int Suppl. 2006; : S4-8.
- Fishbane S, Kowalski EA, Imbriano LJ, Maesaka JK. The evaluation of iron status in hemodialysis patients. J Am Soc Nephrol. 1996; 7: 2654-2657.
- Brugnara C. Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci. 2000; 37: 93-130.
- Fishbane S, Galgano C, Langley RC Jr, Canfield W, Maesaka JK. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997; 52: 217-222.
- Macdougall IC, Cavill I, Hulme B, Bain B, McGregor E, McKay P, et al. Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ. 1992; 304: 225-226.
- Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood. 2002; 99: 1489-1491.
- Brugnara C, Zelmanovic D, Sorette M, Ballas SK, Platt O. Reticulocyte hemoglobin: an integrated parameter for evaluation of erythropoietic activity. Am J Clin Pathol. 1997; 108: 133-142.
- Brugnara C. Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem. 2003; 49: 1573-1578.
- Macdougall IC, Horl WH, Jacobs C, Valderrabano F, Parrondo I, Thompson K, et al. European best practice guidelines 6-8: assessing and optimizing iron stores. Nephrol Dial Transplant. 2000; 15 Suppl 4: 20-32.
- Kotisaari S, Romppanen J, Penttila I, Punnonen K. The Advia 120 red blood cells and reticulocyte indices are useful in diagnosis of iron-deficiency anemia. Eur J Haematol. 2002; 68: 150-156.
- National Kidney Foundation, Kidney Disease Outcomes Quality Initiative NKF-K/DOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006; 47: S11-S145.
- Urrechaga E. Clinical utility of the new Beckman-Coulter parameter red blood cell size factor in the study of erithropoiesis. Int J Lab Hematol. 2009; 31: 623-629.
- Locateli F, Aljama P, Barany P, Canaud B, Carrera F, Eckardt KU, et al. European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19: 1-47.
- Schoorl M, Schoorl M, Nube MJ, Bartels PCM. Erythropoiesis actiity, iron availability and Reticulocyte Hemoglobinization during treatment with Hemodialysis and in subjects with uremia. Clin Lab. 2006; 52: 621-629.
- Bovy C, Gothot A, Delanaye P, Warling X, Krzesinski JM, Beguin Y. Mature erythrocyte parameters as new markers of functional iron deficiency in haemodialysis: sensitivity and specificity. Nephrol Dial Transplant. 2007; 22: 1156-1162.
- Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, et al. The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant. 2001; 16: 1416-1423.
- Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002; 48: 1066-1076.
- Mitsuiki K, Harada A, Miyata Y. Assessment of iron deficiency in chronic hemodialysis patients: investigation of cutoff values for reticulocyte hemoglobin content. Clin Exp Nephrol. 2003; 7: 52-57.
- Kim JM, Ihm CH, Kim HJ. Evaluation of reticulocyte haemoglobin content as marker of iron deficiency and predictor of response to intravenous iron in haemodialysis patients. Int J Lab Hematol. 2008; 30: 46-52.
- d'Onofrio G, Chirillo R, Zini G, Caenaro G, Tommasi M, Micciulli G. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995; 85: 818-823.
- David O, Grillo A, Ceoloni B, Cavallo F, Podda G, Biancotti PP, et al. Analysis of red cell parameters on the Sysmex XE 2100 and ADVIA 120 in iron deficiency and in uraemic chronic disease. Scand J Clin Lab Invest. 2006; 66: 113-120.
- Asanuma M, Taguchi C, Uesaka H, Kumagai T, Seino K, Hosokawa H, et al. Discrepancy between the Percentage of Hypochromic Erythrocytes and the Reticulocyte Hemoglobin Content in Hemodialysis patients with Recombinant Human erythropoietin therapy. Lab Hematol. 2005; 11: 124-130.