
Research Article
Chronic Dis Int. 2015;2(1): 1012.
Suppressive Activity of Macrolide Antibiotics on Periostin Production from Nasal Cells in Vitro and in Vivo
Furuta A¹, Asano K²*, Suzuki T¹, Mizuyoshi T¹, Asano M¹, Kanai K¹ and Kobayashi H¹
1Department of Otolaryngology, Showa University, Japan
2Division of Physiology, Showa University, Japan
*Corresponding author: Kazuhito Asano, Division of Physiology, School of NRS, Showa University, 1865 Touka-Ichiba, Midori-Ku, Yokohama 226-8555, Japan
Received: January 08, 2015; Accepted: March 31, 2015; Published: April 02, 2015
Abstract
Periostin, a 90-kDa extracellular matrix protein, had been attracted attention as a novel biomarker of airway inflammatory diseases such as allergic rhinitis, Chronic Rhinosinusitis (CR) and asthma. Although oral administration of 14-membered macrolide antibiotics into patient with CR could favorably modify the clinical conditions, the influence of macrolide antibiotics on periostin production is not well understood. The present study, therefore, was undertaken to examine the influence of macrolide antibiotics on periostin production in vitro and in vivo. Nasal Polyp Fibroblasts (NPFs) were stimulated with 10.0 ng/ml Interleukin (IL)-13 in the presence of various concentrations of either Clarithromycin (CAM), Roxithromycin (RXM) or Josamycin (JM) for 24 hours. Periostin levels in the culture supernatants were measured by ELISA. Addition of CAM and RXM but not JM into cell cultures caused the suppression of periostin production from NPFs induced by IL-13 stimulation. The minimum concentrations that caused significant suppression were 0.4 μg/ml for CAM and 5.0 μg/ml for RXM. We then examined whether CAM could also inhibit periostin production in vivo. CR patients were orally treated with 200 mg CAM once a day for three months and levels of periostin in nasal secretions was examined by ELISA. Oral administration of CAM caused significant suppression of the appearance of periostin in nasal secretions along with attenuation of clinical symptoms. These results strongly suggest that the ability of CAM to periostin production may account, at least in part, for the clinical efficacy of CAM in CR.
Keywords: Macrolide antibiotic; Clarithromycin; Periostin; Chronic rhinosinusitis; Suppression
Introduction
Low-dose and long-term administration of 14-membered macrolide antibiotic such as erythromycin, Roxithromycin (RXM) and Clarithromycin (CAM) is reported to be able to favorably modify the clinical conditions of Chronic Rhinosinusitis (CR) and chronic lower airway inflammatory diseases, including chronic bronchitis and diffuse panbronchiolitis [1,2]. Long-term administration of 16-membered macrolide antibiotic, Azithromycin (AZ), into patients with Cystic Fibrosis (CF) has been also reported to be able to improve lung functions [3, 4]. Although the efficacy of this macrolide therapy on the chronic airway inflammatory diseases is generally believed to be due to their anti-inflammatory effects rather than anti-bacterial effects [2,5], the precise mechanisms are not well understood.
Histological observations clearly showed that an accumulation of inflammatory cells such as neutrophils and macrophages in the airways is an important future of chronic airway inflammatory diseases such as CR and CF [1,6]. It is also revealed the proliferation and thickening of the mucosal epithelium, with focal squamous metaplasia, glandular hyperplasia, subepithelial fibrosis and stromal edema with numerous blood vessels [7, 8]. These histological changes are called tissue remodeling and these changes are normalized after a period of successful macrolide therapy along with attenuation of clinical conditions of the patients [1,2].
Periostin, a nonstructural matricellular protein, is a 90-kDa extracellular matrix protein secreted from various types of cells, including fibroblasts, epithelial cells and other structural cells after IL-4/IL-13 stimulation [7-9]. Periostin is reported to increase collagen synthesis and fibrillogenesis by binding to collagen and enhancement of regulation of TGF-β signaling [10,11]. It is also reported that periostin increases the ability of airway fibroblasts to produce chemokines responsible for the recruitment of inflammatory cells, especially neutrophils and macrophages [11,12], which are essential final effector cells in the development of CR and CF [8,13]. Furthermore, periostin strongly promotes the secretion of Matrix Metalloproteinases (MMP), MMP-2 and -9 from macrophages [14], which are involved in the induction of both tissue remodeling and degradation of extracellular matrix in CR [13]. Although periostin had been attracted attention as a novel biomarker of airway inflammatory diseases such as allergic rhinitis, CR and asthma [7,8,10-12], the influence of macrolide antibiotics on periostin production is not well understood. The present study, therefore, was undertaken to examine the influence of macrolide antibiotics on periostin production in vitro and in vivo.
Materials and Methods
Reagents
CAM and RXM, preservative free pure powders, were kindly donated by Taisho-Toyama Pharmaceutical Co. Ltd. (Tokyo, Japan) and Sanofi Aventis Pharmaceutical Co. Ltd. (Tokyo, Japan), respectively. These agents were dissolved firstly in 100% ethyl alcohol at a concentration of 10.0 mg/ml and then diluted with Phosphate- Buffered Saline (PBS) at a concentration of 10 μg/ml. These were sterilized by passing through 0.2 μm filters, and stored at 4°C until used. Josamycin (JM) purchased from SIGMA Pure Chemical Co. (St Louis, MO, USA) was also dissolved in PBS in a similar manner. Just before use, these agents were further diluted with RPMI-1640 medium (SIGMA Pure Chemical Co.) supplemented with 10% Heat Inactivated Fetal Calf Serum (RPMI-FCS; Irvine Scientific Co., Ltd, Santa Ana, CA, USA) at appropriate concentrations. Recombinant Human Interleukin (IL)-13 was purchased from R & D Systems Inc. (Minneapolis, MN, USA).
Subjects and treatment
The subjects were 25 patients (male 15; female 10) with CR and 10(male 5; female 5) healthy subjects. Male subjects were recruited from the Otolaryngology Outpatient Clinic of Sasaki Hospital (Yokohama, Japan) and female subjects were from Showa University Hospital (Tokyo, Japan) with a written informed consent approved by the Ethics Committee of Showa University (Tokyo, Japan). CR was diagnosed by Otorhinolaryngologists in accordance with the established criteria on the basis of patient history, rhinoscopic and X-ray examination. The characteristics of the CR patients used in this study are shown in Table 1. CR patients were orally treated with 200 mg CAM once a day for three months [1,2,6].
Controls
Patients
Male
Age, years(range)
Number of subjects
Bronchial asthma
Nasal polyposis
Disease severity
Medication
50-59
5
None
None
None
None
54-65
15
None
None
Sever
None
Female
Age, years(range)
Number of subjects
Bronchial asthma
Nasal polyposis
Disease severity
Medication
48-53
5
None
None
None
None
52-63
10
None
None
Sever
None
Table 1: Characteristics of subjects used in the study.
Recovery of nasal secretions
Nasal secretions were obtained from patients before and after treatment with CAM and healthy subjects as previously described [15]. Briefly, a filter strip (Whatman No.42; 7 x 30 mm) was placed on the anterior portion of the inferior turbinate of the right and left nose and left for 5 min. They were then cut into small pieces, suspended in PBS and rocking for 12 h at 4°C to prepare the extract of nasal secretions. After measuring IgA concentration in the extract with ELISA (Bethyl Laboratories, Inc., Montgomery, TX, USA) according to the manufacturer’s recommendations, they were stored at –80°C until used.
Nasal symptom scores
Clinical condition of patients was evaluated by subjective symptoms (nasal discharge, obstruction and postnasal discharge), objective findings (nasal discharge, postnasal discharge and swelling of the nasal mucosa) and X-ray findings (Waters view and Caldwell view) before and after treatment. Symptoms and findings were scored from 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = sever symptoms).
Cell source
Nasal polyp specimens were obtained from patients given a written informed consent, during surgical operations, according to the protocol approved by the Ethics Committee of Showa University. Specimens were diced into small pieces (approximately 1 mm in size), washed several times in PBS supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin and 5.0 μg/ml amphotericin B, followed by antibiotics free RPMI-FCS. Diced specimens were then plated at a density of 10 pieces in 100-mm tissue culture dishes and covered with a cover slip adhered to the dish with sterile vaseline. The dishes were then placed at 37°C in a humidified atmosphere with 5% CO2. When a monolayer of fibroblast-like cells was found to be confluent, the explanted tissues were removed. The cells were then trypsinized and replated at a concentration of 5x105 cells/ml. The medium was changed every 3 days for 2-3 weeks until confluence was attained. Subsequently, the cells were split into 1:2 at confluency and passaged. The cells were characterized [16], and used as Nasal Polyp Fibroblasts (NPFs).
Cell culture
The cells, passaged between four to seven, were washed several times with RPMI-FCS, introduced into each well of 24-well culture plates at a concentration of 1x105 cells/ml in a volume of 1.0 ml and allowed to adhere for 24 h. The plates were then washed twice with RPMI-FCS to removed dead and un-attached cells. The cells were stimulated with various concentrations of IL-13 in a total volume of 2.0 ml. After 24 h, the supernatant was obtained and stored at –80°C until used. In cases of examining the influence of macrolide antibiotics, CAM, RXM and JM on the production of periostin, the cells were cultured with 10.0 ng/ml IL-13 in the presence of various concentrations of macrolide antibiotics for 24 h. The culture supernatants were obtained and stored at –80°C until used.
Assay for periostin
Periostin levels in culture supernatants were examined by the commercially available periostin ELISA test kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) according to the manufacturer’s recommendations. Periostin levels in nasal secretions was also measured by the commercially available periostin ELISA test kits (Phoenix Pharmaceuticals, Inc.) in a similar manner and the results were expressed as the mean ng/ng IgA ± SE. The minimum detection levels of periostin ELISA test kit was 0.027 ng/ml.
Statistical analysis
Statistical significance between control and experimental groups was examined by ANOVA followed by Dunette’s multiple comparison tests. Paired t-test was used to examine the statistical significance between before and after treatment with CAM. Data analysis was performed by using ANOVA for Mac (SPSS Inc., Chicago, IL, USA). The level of significance was considered at a P value of less than 0.05.
Results
Influence of macrolide antibiotics on periostin production from nasal fibroblasts after IL-13 stimulation
The optimal concentration of IL-13 for stimulation of NPFs to produce periostin was determined in an initial experiment. NPFs (1x105 cells/ml) were stimulated with various concentration of IL- 13 in triplicate and culture supernatants were collected 24 h after culture for measurement of periostin concentration by ELISA. As shown in Figure 1, IL-13 stimulation caused increase in periostin production from NPFs, which was firstly observed at 1.0 ng/ml and peaked at more than 5.0 ng/ml. The next experiments were designed to examine whether macrolide antibiotics could suppress periostin production from NPFs in response to IL-13 simulation. NPFs (1x105 cells/ml) were stimulated with 10.0 ng/ml IL-13 in the presence of various concentrations of CAM, RXM or JM for 24 h, and periostin levels were examined by ELISA. As shown in Figure 2A, addition of CAM into cell cultures caused the suppression of the ability of NPFs to produce periostin induced by IL-13 stimulation. The minimum concentration of CAM that caused significant suppression was 0.4 μg/ml. It is also showed that RXM at more than 5.0 μg/ml could inhibit the ability of nasal fibroblasts to produce periostin in response to IL-13 stimulation (Figure 2B). On the other hand, JM could not suppress the production of periostin after IL-13 stimulation, even when 15 μg/ml JM was added to cell cultures: periostin levels in culture supernatant from cells treated with 15 μg/ml JM was nearly identical (not significant; p > 0.05) to that in control supernatants (Figure 2C).
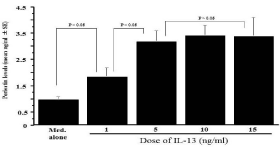
Figure 1: Influence of IL-13 stimulation on periostin production from NPFs
in vitro. Nasal Polyp Fibroblasts (NPFs) at 1x105 cells/ml were cultured in
the presence of various concentrations of IL-13 for 24 h. Concentration of
periostin in culture supernatants was measured by ELISA and the results
were expressed as the mean ng/ml ± SE of five different subjects.
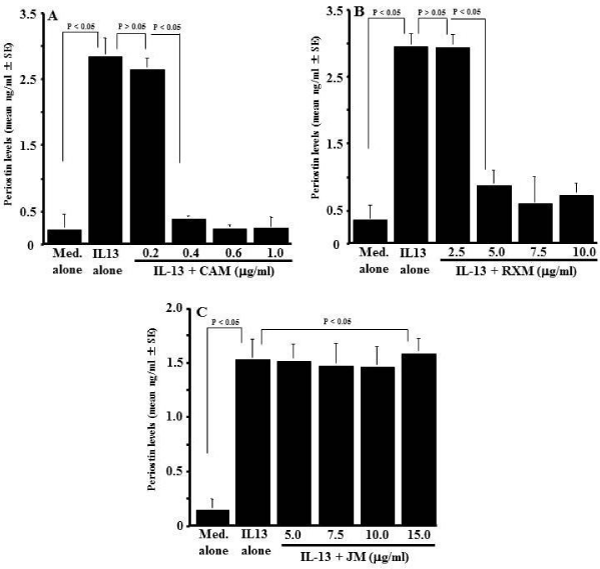
Figure 2: Influence of macrolide antibiotics on periostin production from
NPFs after IL-13 stimulation in vitro. Nasal Polyp Fibroblasts (NPFs) at
1x105cells/ml were cultured with 10.0ng/ml IL-13 for 24 hours in the presence
of various concentrations of Clarithromycin (CAM), Roxithromycin (RXM)
or Josamycin (JM). Periostin concentration in culture supernatants were
measured by ELISA and the results were expressed as the mean ng/ml ± SE
of five different subjects. A: CAM; B: RXM; C: JM.
Influence of CAM treatment on periostin appearance in nasal secretions from CR patients
The third experiments were designed to examine the influence of CAM on the appearance of periostin in nasal secretions. To do this, CR patients were orally administered 200 mg CAM once a day for three months, and periostin levels in nasal secretions were examined by ELISA. As shown in Figure 3A, oral administration of CAM into male patients caused significant decrease in periostin levels in nasal secretions as compared with before treatment. This suppressive activity of CAM on periostin production was also observed in female patients with CR (Figure 3B).
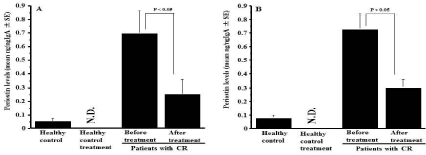
Figure 3: Influence of clarithromycin treatment on the appearance of periostin
in nasal secretions obtained from patients with chronic rhinosinusitis. Patients
with chronic rhinosinusitis were treated with 200 mg clarithromycin once a
day for three months. Periostin levels in nasal secretions were examined by
ELISA and the results were expressed as the mean ng/ng IgA ± SE. The
number of subjects was 10 (Female: 5, Male: 5) for healthy control and 25
for patients (Female: 10; Male: 15) with chronic rhinosinusitis. A: Male; B:
Female; N.D.: Not done.
Influence of CAM treatment on clinical symptoms in CR patients
The final experiments were performed to examine whether oral administration of CAM could favorably modify the clinical conditions of CR patients. As shown in Table 2, both nasal symptoms and findings in CR patients were significantly improved by the macrolide therapy. X-ray findings were also improved by the macrolide therapy (Table 2).
Before treatment
After treatment*
Male
Subjective symptoms
Nasal discharge
Nasal obstruction
Postnasal discharge
Objective finding
Swelling of the nasal mucosa
Nasal discharge
Postnasal discharge
X-ray findings
1.67 ± 0.9
0.93 ± 0.25
1.60 ± 0.25
1.60 ± 0.16
1.47 ± 0.13
0.80 ± 0.14
3.33 ± 0.39
0.47 ± 1.19
0.27 ± 0.12
0.40 ± 0.19
0.67 ± 0.19
0.73 ± 0.15
0.13 ± 0.09
1.60 ± 0.29
Female
Subjective symptoms
Nasal discharge
Nasal obstruction
Postnasal discharge
Objective finding
Swelling of the nasal mucosa
Nasal discharge
Postnasal discharge
X-ray findings
1.83 ± 0.41
0.67 ± 0.33
1.71 ± 0.31
1.83 ± 0.31
1.67 ± 0.21
0.83 ± 0.33
2.67 ± 0.71
0.50 ± 0.22
0.17 ± 0.17
0.17 ± 0.17
0.83 ± 0.31
0.83 ± 0.31
0
1.33 ± 0.42
*Statistically significant as compared with before treatment.
Table 2: Changes in clinical symptom scores of patients with chronic rhinosinusitis before and after treatment with clarithromycin.
Discussion
Low-dose and long-term administration of macrolide antibiotics favorably modify the clinical conditions of the patients with inflammatory airway diseases, when the patient was administered 14- and 16-membered, but not 15-membered macrolide antibiotics [1- 4]. Although there are much evidence that anti-inflammatory action of macrolide antibiotics, such as the suppression of inflammatory cytokine productions and polymorphoneuclear leukocyte activations, may underlay the therapeutic mode of action of macrolides [1,2,5,6], the precise mechanisms of macrolide therapy are not well understood.
It is now accepted that body functions maintained a constant stability even when it exposed to fluctuating conditions in the external environment. This is called homeostasis and regulated by the endocrine system and the endogenous peptides after several stimuli, as well as the sympathetic nervous system. Among these, the endogenous peptides, especially periostin attracted attention as an important endogenous peptide in the development of chronic airway inflammatory diseases [7-12]. In the present study, therefore, we examined the influence of macrolide antibiotics, especially CAM, RXM and JM on the production of periostin from nasal cells in vitro and in vivo. The present results clearly showed that CAM and RXM but not JM could suppress the ability of NPFs to produce periostin in response to IL-13 stimulation in vitro.
Periostin is originally identified in mouse osteoblasts as a cell adhesion protein and is recently classified into a multicellular matrix protein belonging to the fascilin family [7,8,11]. Periostin is expressed in airway epithelial cells and lung fibroblasts [9,17]. Experimental and clinical evidences clearly indicate that periostin derived from epithelial cells and fibroblasts is responsible for the development of airway inflammatory diseases, including CR, as a component in subepithelial fibrosis [7,8,18]. Although periostin is recognized to play essential roles in wound healing and myocardial repair [11,19], its overproduction in the nasal mucosa has been reported to contribute to polyp formation, mucus hyper-production and edema in nasal mucosa [7, 8], which lead to obstruction of the sinonasal passages observed in CR patients [8]. In experimental mouse model of CR, intranasal instillation of ovalbumin into periostin-deficient mice is reported to produce much lower numbers of polyp-like lesions as compared with wild type mice [18]. After oral administration of CAM into human, plasma levels of the agent gradually increases and plateau at 1.0±0.16 μg/ml [5], which are much higher levels that caused suppressive activity on periostin production from nasal fibroblasts in vitro. Taken together, the present results may suggest that oral administration of 14-membered macrolide antibiotics, especially CAM into CR patients caused decrease in the ability of nasal cells to produce periostin and result in favorable modification of clinical conditions of patients. To further confirm this speculation, we then examined the influence of CAM on the appearance of periostin in nasal secretions obtained from CR patients treated with CAM for three months. The present results clearly showed that nasal secretions obtained from CR patients before treatment contained much higher levels of periostin as compared with those from healthy control, and that oral administration of CAM into CR patients caused decrease in periostin levels in nasal secretions along with attenuation of clinical conditions, suggesting that suppressive activity of CAM on periostin production from nasal cells may constitute, at least in part, of the therapeutic mode of action of macrolide antibiotics in CR.
IL-13 is well accepted to be a pleotrophic inflammatory cytokine mainly secreted from TH2 type T cells [20] and has been shown to be central to the pathogenesis of airway inflammatory diseases, including CR [21,22]. It is also accepted that IL-13 activates several components implicated in cellular signal transduction [20]. IL-13 first attaches to IL-13 receptor alpha (Ra) 1 subunit, which then leads to subsequent recruitment of IL-4 Ra chain. Binding of IL-13 to IL-13Ra/IL-4Ra complex induces the activation of several signal transduction pathway such as Janus kinases 1 (JAK1) and tyrosine kinase 2 [23]. Activation of these tyrosine kinases then causes the phosphorylation and activation of several types of transcription factor, which is essential for inflammatory protein production, including cytokines and MMPs [14,24]. 14- and 16-membered macrolide antibiotics, including CAM, RXM and AZ had been reported to inhibit the phosphorylation of several types of tyrosine kinases induced by inflammatory stimulation [2,25,26]. Rapamycin classified into macrolide antibiotics is also reported to exert suppressive effect on phosphorylation of tyrosine kinases, resulting in inhibition of the development of several types of immune responses responsible for graft rejection [27]. On the other hand, 15-membered macrolide antibiotic, JM could not suppress phosphorylation of tyrosine kinases after lipopolysaccharide stimulation in airway epithelial cells and results in increase in both nuclear translocation of transcription factors, especially NF-κB and inflammatory protein production induced by inflammatory stimulation [25]. These reports may suggest that CAM and RXM but not JM inhibits the phosphorylation of tyrosine kinases induced by IL-13 stimulation, and results in suppression of periostin production from nasal cells. Further experiments are needed to clarify the suppressive mechanisms of macrolide antibiotics on periostin production from nasal cells after inflammatory stimulation.
References
- Keichou N, Kudoh S. Diffuse panbronchiolitis: role of macrolides in therapy. Am J Respir Med. 2002; 1: 119-131.
- Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immuno modulatory medications: Proposed mechanisms of action. Pharmacol Thepeutics. 2008; 117: 393-405.
- Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet. 1998; 315: 420.
- Equi A, Balfour-Lynn IM, Bush A, Rosental M. Long-term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trail. Lancet. 2002; 360: 978-984.
- Furuya A, Asano K, Shoji N, Hirano K, Hamasaki T, Suzaki H. Suppression of nitric oxide production from nasal fibroblasts by metabolized clarithromycin in vitro. J Inflamm. 2010; 7: 56.
- Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol Suppl. 1992; 157: 16-20
- Ohta N, Ishida A, Kurakami K, Suzuki Y, Kakehata S, Ono J, et al. Expressions and roles of periostin in otolaryngological diseases. Allergol Int. 2014; 63: 171-180.
- Ishida A, Ohta N, Suzuki Y, Kakehara S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012; 61: 589-595.
- Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; 118: 98-104.
- Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014; 71: 1279-1288.
- Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a multicellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012; 46: 677-686.
- Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014; 37:150-156.
- Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro. Eur Respir J. 2004; 23: 671-678.
- Hakuno D, Kimura N, Mukai M, Kimura T, Okada Y, Yozu Y, et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010; 120: 2292-2306
- Nogaki T, Asano K, Furuta A, Kanai K, Suzaki I, Kanei A, et al. Enhancement of clara cell 10-kD protein (CC10) production from nasal epithelial cells by fexofenadine hydrochloride. Asian Pac J Allergy Immunol. 2012; 30: 139-145.
- Asano K, Kamakazu K, Hisamitsu T, Suzaki H. Suppressive activity of macrolide antibiotics on nitric oxide production from nasal polyp fibroblasts in vitro. Acta Otolaryngol. 2003; 123: 1064-1069.
- Shoda T, Futamura K, Kobayashi F, Saito H, Matsumoto K, Matsuda A. Cell type-dependent effects of corticosteroid on periostin production by primary human tissue cells. Allergy. 2013; 68: 1467-1470.
- Kim SW, Kim JH, Jung MH, Hu DG, Lee HK, Jeon SY, et al. Periostin may play a protective role in the development of eosinophilic chronic rhinosinusitis with nasal polyps in a mouse model. Laryngoscope. 2013; 123: 1075-1081.
- Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithlialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011; 6: 18410.
- Corren J. Role of interleukin-13 in asthma. Cur Allergy Asthma Rep. 2013; 13: 415-420.
- Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188: 432-439.
- Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013; 131: 772-780.
- Chiba Y, Goto K, Misawa M. Interleukin-13-induced activation of signal transducer and activator of transcription 6 is mediated by an activation of Janus kinase 1 in cultured human bronchial smooth muscle cells. Pharmacol Rep. 2012; 64: 454-458.
- Shoji N, Asano K, Furuta A, Hirano K, Suzaki H. Effect of histamine H1 receptor antagonists on TARC/CCL17 and MDC/CCL22 production from CD14+ cells induced by antigenic stimulation in vitro. Int Arch Allergy Immunol. 2011; 155: 38-51.
- Ou XM, Feng YL, Wen FQ, Wang K, Yang J, Deng ZP, et al. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-κB. Respirology. 2008; 13: 63-72.
- Morinaga Y, Yanagihara K, Miyashita N, Seki M, Izumikawa K, Kakeya H, et al. Azithromycin, clarithromycin and telithromycin inhibit MUC5AC induction by Chlamydophila pneumonia in airway epithelial cells. Pulmonary Pharmacol Thera. 2009; 22: 580-586.
- Liu Y, Sun SY, Owonikoko TK, Sica GL, Curran WJ, Khuri FR, et al. Rapamycin induces bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther. 2012; 11: 1535-7163.