
Review Article
Austin J Clin Neurol 2015;2(3): 1031.
Clinical Features, Diagnosis, and Treatment of Poststroke Cognitive Impairment
Kazuo Washida1*, Hisatomo Kowa1, Hirotoshi Hamaguchi2, Hisatsugu Tachibana1, Kenji Sekiguchi1, Fumio Kanda1 and Tatsushi Toda1
1Division of Neurology, Graduate School of Medicine, Kobe University, Japan
2Department of Neurology, Kita-harima Medical Center, Japan
*Corresponding author: Kazuo Washida, Division of Neurology, Kobe University Graduate School of Medicine, Kusunoki-cho 7-5-2, Chuo-ku, Kobe 650-0017, Japan
Received: December 22, 2014; Accepted: March 14, 2015; Published: April 02, 2015
Abstract
Poststroke Cognitive Impairment (PSCI) is becoming a major burden in our aging society, and its prognosis is poor, even compared with patients with Alzheimer’s disease. Executive dysfunction and working memory impairment are characteristic symptoms of PSCI. Additionally, poststroke apathy, which is a difficult neuropsychiatric sequela, often afflicts stroke survivors and is an important obstacle for rehabilitation.
For PSCI detection, the Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) because the MoCA assesses a number of executive functions with visuoexecutive, abstraction, and conflicting instructions items. Additionally, the Apathy Scale is a useful tool for the early detection of poststroke apathy.
Recently, the ASCO phenotypic classification of stroke has been proposed as a new classification system of stroke. The ASCO classification evaluates the etiology and mechanisms of ischemic stroke comprehensively and systematically. In addition, the risk factors for cognitive impairment, such as arterial sclerosis, leukoaraiosis, and atrial fibrillation, can be assessed simultaneously and graded with the ASCO classification. The total score of the ASCO classification correlates significantly with the total scores on the MoCA and MMSE. The ASCO classification is useful for assessing both the etiology of ischemic stroke and cognitive decline in stroke survivors.
Both medication therapy, with agents such as acetylcholinesterase inhibitors, and cognitive rehabilitation therapy are considered effective treatments for patients with PSCI.
In this review, the clinical features, diagnosis, and treatment of patients with PSCI are extensively described to aid in early detection and treatment, which will improve the prognosis of these patients.
Keywords: Poststroke Cognitive Impairment; Poststroke apathy; Montreal Cognitive Assessment; Apathy Scale; ASCO phenotypic classification of stroke
Abbreviations
PSCI: Poststroke Cognitive Impairment; MCI: Mild Cognitive Impairment; MoCA: Montreal Cognitive Assessment; MMSE: Mini- Mental State Examination; BPSD: Behavioral and Psychological Symptoms of Dementia
Poststroke Cognitive Impairment
The burden of stroke that results from its effects on cognition has been underestimated for a long time. Ischemic stroke is a major cause of adult chronic disabilities such as motor and sensory disturbances, and it represents an important cause of cognitive decline and dementia [1]. Vascular cognitive impairment is the second leading cause of dementia after Alzheimer’s disease. About one-third of stroke survivors suffer from Poststroke Cognitive Impairment (PSCI) [1]. The prevalence of cognitive impairment among patients with a history of stroke is similar to that of subjects 10 years older without a history of stroke [2]. A recent study demonstrated that poststroke mild cognitive impairment (MCI) is progressive and develops into dementia in 24.4% of patients within 3 years, resulting in a mean conversion rate of approximately 8% per year [2]. Thus, the concept of PSCI, including poststroke MCI, should be understood by clinicians so that it is detected early and treated, even if the impairment is not severe enough to meet the criteria for dementia. This review focuses on the clinical features, diagnosis, and treatment of patients with PSCI in order to execute early detection and treatment, which will improve the prognosis of these patients.
Clinical features
PSCI is defined as cognitive impairment that occurs within 3 months after stroke [3]. This definition is based on the fact that pathological changes after strokes usually converge within 3 months after the stroke. PSCI exhibits relatively uniform clinical and pathological features, and it is mainly comprised of two subtypes: multi-infarct dementia and strategic single-infarct dementia.
Slowing of processing speed and working memory impairment
PSCI is mainly composed of multi-infarct dementia that is caused by lacunae, atherosclerosis, and embolisms [4]. Most patients who experience cerebral infarctions involving the white matter exhibit a slowing of processing speed in cognitive functions, such as executive function, calculation, and abstraction. The white matter, which is the main component of the frontal-subcortical circuits of the brain, is strongly associated with processing speed [5]. Working memory impairment is a characteristic feature of PSCI because working memory depends on white matter integrity [5]. Furthermore, patients with PSCI often exhibit mild recent memory impairment because of damage to the white matter that intimately connects with the hippocampus, and this damage can indirectly induce hippocampal atrophy through remote effects [5].
Strategic single-infarct dementia
Strategic single-infarct dementia is caused by a single infarction in key functional areas of the brain, such as the thalamus, anterior cerebral artery-territorial area, and angular gyrus [6]. The sudden onset of focal cognitive impairment, such as apraxia and apathy, strongly suggests strategic single-infarct dementia. The clinical features depend on the site of infarction. An anterior thalamic infarction results in sudden onset of impaired consciousness, aphasia, and apathy. A hippocampal infarction results sudden severe recent memory disturbances. Focal cerebral cortical symptoms, such as aphasia, apraxia, and agnosia, are also characteristic disturbances of strategic single-infarct dementia.
Poststroke apathy, depression, and emotional incontinence
Patients who have suffered a stroke often experience mood changes. Behavioral and Psychological Symptoms of Dementia (BPSD) are a series of very important symptoms of dementing illness. BPSD is the major cause of caregiver’s exhaustion, and these symptoms can be improved by drug therapy, rehabilitation therapy, and environmental arrangements. Apathy, depression, irritation, anger, and delusions of persecution are well-known symptoms of BPSD. Especially, apathy is a very troublesome sequela that often leads to caregiver exhaustion [7]. Poststroke apathy is a disturbance of motivation that is exhibited by low self-activation or emotional indifference. Frontal lobe hypoactivity is hypothesized to contribute to the development of poststroke apathy, and stroke survivors who are older and who have poorer cognitive status and frontal dysfunction tend to exhibit apathetic symptoms [7]. Apathy often results in disuse syndrome. However, poststroke apathy has received little attention in daily clinical practice despite the fact that it is a major reason for a poor poststroke prognosis.
Poststroke depression, which is one of the common BPSD, has been reported to occur in 33-56% of patients after a stroke, and it is associated with worse functional outcome, slower recovery, and poorer quality of life after the stroke [1]. Furthermore, poststroke emotional incontinence, such as anger and crying, is one of the important positive BPSD that often induces serious problems and leads to severe caregiver exhaustion. Emotional incontinence is a neurological condition characterized by uncontrollable episodes of emotions such as anger; it occurs within 1 year after a stroke with a prevalence of approximately 20%. It leads to poorer quality of life of both patients and caregivers [8].
Diagnosis
PSCI is usually diagnosed when a cognitive impairment is related to the onset of a stroke. Clinicians should pay attention to the timespace relationship between the onset of cognitive impairment and the stroke. In addition to advances in imaging techniques, such as single-photon emission computed tomography and diffusion tensor imaging, simple cognitive screening tests, such as the Montreal Cognitive Assessment (MoCA) and the Apathy Scale, have good sensitivity and specificity in detecting PSCI. Recently, the ASCO phenotypic classification of stroke has been reported to be useful for assessing both the etiology of ischemic stroke and cognitive decline in stroke survivors.
Definition of poststroke cognitive impairment
The National Institute of Neurological Disorders and Stroke- Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria defines PSCI as cognitive impairment that occurs within 3 months after a stroke [3]. PSCI is diagnosed when there is a strong connection between cognitive impairment and stroke. In daily clinical practice, the presence of stroke is usually diagnosed with neuroimaging modalities, such as brain magnetic resonance imaging and computed tomography, and simple cognitive screening tests, such as the MoCA, the Mini-Mental State Examination (MMSE), and the Apathy Scale, are useful for detecting PSCI.
Montreal cognitive assessment
Several batteries for neuropsychological evaluations are available for predicting PSCI. Although the MMSE has been used in daily clinical practice and is well-validated, it tends to overlook executive dysfunction and is not a suitable tool for detecting PSCI. In order to detect cognitive impairments in stroke survivors, the MoCA is superior to the MMSE [9]. The MoCA is a simple and stand-alone cognitive screening test with good sensitivity and specificity in detecting PSCI, and it can evaluate a number of executive functions, such as visuoexecutive functions, abstraction, similarities, and conflicting instructions, more sensitively than the MMSE can. Pantoni et al. have also reported that the MoCA score at the acute phase of an ischemic stroke can predict the future cognitive ability of a stroke survivor [10].
Apathy scale
Poststroke apathy acts as a barrier to meaningful participation in cognitive and physical rehabilitation [11]. The Apathy Scale, which is useful for detecting poststroke apathy, is a simple, questionnairetype, cognitive screening test with good sensitivity and specificity in detecting poststroke apathy. The Apathy Scale has more than 16 points (Total score: 42 points) that concern the apathetic state. The Apathy Scale has been validated in many clinical trials, and it accurately reflects the temporal changes in poststroke apathy [7].
ASCO phenotypic classification of stroke
The ASCO classification of stroke, which has been proposed by Amarenco et al., and evaluates the etiology and mechanisms of ischemic stroke more comprehensively and systematically than conventional stroke classification systems such as the Trial of Org 10172 in Acute Stroke Treatment (TOAST) system [12]. The risk factors for cognitive impairment, such as arterial sclerosis, leukoaraiosis, and atrial fibrillation, can be assessed simultaneously and graded with the ASCO classification (Figure 1). We have recently reported that the total score on the ASCO classification significantly correlates with the total scores on the MoCA and MMSE [13] (Figure 2). This correlation was more apparent for the MoCA than for the MMSE because the MoCA scores were normally distributed, whereas the MMSE scores were skewed toward the higher end of the range (ceiling effect). For example, one patient suffering from a small lacunar infarction and having a low ASCO score of 3/12 (A0- S3-C0-O0) showed normal cognitive function (MoCA score: 27/30; MMSE score: 30/30). However, the other patient who was suffering from an atherothrombotic brain infarction and had a high ASCO score of 9/12 (A3-S2-C3-O1; Figure 1) showed cognitive impairment (MoCA score: 10/30; MMSE score: 15/30). As a result, we were able to detect the cognitive impairment early and treat it, which improved the prognosis of patients with PSCI. These results suggest that the ASCO classification of stroke is useful both for assessing the etiology of ischemic stroke and for predicting cognitive decline after ischemic stroke.

Figure 1: Example of the ASCO grading system (phenotyping) in a patient
with an ipsilateral carotid stenosis > 50% (atherosclerosis, grade 3), a single,
deep branch artery stroke (small vessel disease, grade 2), atrial fibrillation
(cardioembolism, grade 3), and a platelet count of 700,000/mm3 (other
causes, grade 1). Stroke subtype is A3-S2-C3-O1.
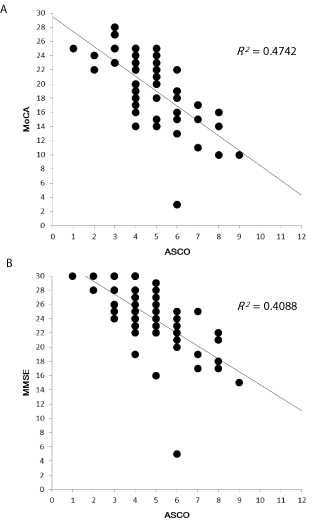
Figure 2: Distribution of MoCA-J, MMSE, and ASCO scores of patients after ischemic stroke. A significant correlation exists between the ASCO phenotypic classification total score and MoCA-J (R2 = 0.47; P < 0.05) (A). There is also a significant correlation between the ASCO phenotypic classification total score and the MMSE score (R2 = 0.41; P < 0.05) (B) (Pearson correlation analysis). Compared to MoCA-J scores, MMSE scores are skewed toward the higher end of the range (ceiling effect).