
Research Article
Austin J Clin Neurol 2015;2(6): 1053.
Ameliorative Treatment with Ellagic Acid in Scopolamine Induced Alzheimer’s Type Memory and Cognitive Dysfunctions in Rats
Ramandeep Kaur, Sidharth Mehan*, Deepa Khanna and Sanjeev Kalra
Department of Pharmacology, Rajendra Institute of Tech & Sciences, India
*Corresponding author: Sidharth Mehan, Associate Professor, Department of Pharmacology, Rajendra Institute of Tech & Sciences, Sirsa-125055, Haryana, India
Received: March 19, 2015; Accepted: May 14, 2015; Published: May 29, 2015
Abstract
Objective: To determine neuroprotective effects of Ellagic acid (EA) as a preventive herbal drug to impede cholinergic dysfunctions and oxidative stress in Alzheimer’s disease (AD) in scopolamine induced Alzheimer’s type dementia in rats.
Methodology: Alzheimer’s type dementia was induced by intraperitoneal injection of scopolamine (0.7 mg/kg, i.p.) to rats for period of 7 days. EA (25 mg/kg or 50 mg/kg, p.o.) or Donepezil (0.5 mg/kg, p.o.) alone was treated for 6 days and then scopolamine (0.7 mg/kg, i.p.) was administered together with EA or Donepezil for another 7 days. Memory-related behavioral parameters were evaluated using the elevated plus maze (EPM) once a day for 2 consecutive days and Morris water maze (MWM) once a day for 5 consecutive days. At the end of protocol schedule i.e day 14, biochemical parameters were estimated. AChE, MDA, GSH, catalase and SOD to evaluate the neuroprotective action of EA via AChE inhibition and antioxidant activity.
Result: Scopolamine treatment increased the transfer latency in EPM, escape latency time and shortened time spent in the target quadrant in MWM; these effects were reversed by EA. Scopolamine-mediated changes in malondialdehyde (MDA) and AChE activity were significantly attenuated by EA in rats. Recovery of antioxidant capacities, including reduced glutathione (GSH) content, and the activities of SOD and catalase was also evident in EA treated rats.
Conclusion: The present findings sufficiently encourage that EA has a preventive properties. Although EA was found to be less effective than Donepezil, but few modification in pharmaceutical properties it can be an efficient phytochemical for Alzheimer type dementia. The EA can be used to prevent cholinergic dysfunctions and oxidative stress associated with Alzheimer type dementia.
Keywords: Neuroinflammation; Oxidative stress; Acetylcholinesterase; Polyphenols
Introduction
Alzheimer’s disease (AD) is a severe neurodegenerative disorder that gradually results in loss of memory and impairment of cognitive functions in the elderly [1,2].
Many naturally occurring compounds have been proposed as potential therapies to slow or prevent the progression of AD, mostly by acting as antioxidants [3-5], but also with some direct anti-amyloid actions [6-8]. Recent studies have suggested the positive effects of dietary antioxidants as an aid in potentially reducing somatic cell and neuronal damage by free radicals [3,9,10]. The beneficial health effects of plant-derived products have been largely attributed to polyphenolic compounds, as well as vitamins, minerals and dietary fibers [3,4,11].
Ellagic acid (EA), a non flavonoid polyphenol, plays an essential role in explaining the pharmacological properties of fruits, food and beverages which exhibit this phyto-constituent [12-14]. EA has been well proven to contain anti-oxidant [15-17], anti-inflammatory [18,19], anti-proliferative [20-22], antidiabetic [23,24] and cardioprotective [25,26] properties.
Neuroprotection can be a property of EA as it prevents both neuro-oxidation and neuroinflammation [27-30]. Moreover, in invitro studies it was observed that EA inhibits β-secretase (BACE1), thus inhibiting Aβ-fibrillation and decrease AChE activity [31- 33]. Recent studies suggested that glucose metabolism is affected during AD [34]. The EA stimulated GLUT4 translocation primary factor responsible for insulin induced glucose uptake and maintain glucose homeostasis [35,36]. The EA also shows modulation of monoaminergic system (serotonergic and noradrenergic systems) and GABAnergic system [37,38]. Cognitive impairment in AD patients correlates with disturbance in various neurotransmitters, as the ratio of excitatory-inhibitory neurotransmitter level disturbs, cytotoxic damage to neurons and glia occurs and norepinephrine and serotonin levels declined [39-43]. Further, Gamma-amino butyric acid (GABA) increases the formation of soluble receptor for advanced glycation end products (RAGE) and decreases the levels of full-length RAGE, lowering the Aβ uptake and inflammatory mediated reactions [44,45].
Scopolamine, an anti-muscarinic agent, competitively antagonizes the effect of acetylcholine on the muscarinic receptors by occupying postsynaptic receptor sites with high affinity and increases AChE activity in the cortex and hippocampus [46-50]. Scopolamine diminish cerebral blood flow due to cholinergic hypofunction [51,52]. Scopolamine additionally triggers ROS, inducing free radical injury and an increase in a scopolamine-treated group brain MDA levels and deterioration in antioxidant status [53-55]. Scopolamine induces neuro-inflammation by promoting high level of oxidative stress and pro inflammatory cytokines in the hippocampus [56- 58]. Scopolamine is proven to increase levels of APP and Tau. Administration of scopolamine led to marked histopathological alterations in the cerebral cortex, including neuronal degeneration [59,60]. Scopolamine administration has been used both in healthy human volunteers and in animals as a model of dementia to determine the effectiveness of potential new therapeutic agents for Alzheimer’s disease [61-66] (Figure 1).

Figure 1: Scopolamine induced experimental model of Alzheimer’s type
dementia.
Donepezil is a well established drug for clinical treatment and scopolamine induced Alzheimer type dementia. Donepezil, a reversible inhibitor of AChE, is neuroprotective due to not only activation of cholinergic transmission but also by reducing the amount of the toxic form of amyloid β fibrils [67-71]. Donepezil ameliorated the scopolamine induced memory impairment by reducing AChE activity and oxidative stress and restoring cerebral circulation [72- 74]. With this background, EA might show neuroprotection via inhibiting neuronal dysfunctions. This research was an attempt to investigate the neuroprotective effect of EA, potential of doses for the prevention of Alzheimer’s disease.
Material and Method
Chemicals
EA was purchased from Yucca Interprises, Mumbai, India and suspended in saline solution. Scopolamine hydrochloride was purchased from Sigma–Aldrich, St, Louis, MO, USA. Donepezil was obtained from Ranbaxy Pvt. Limited, Mumbai, India and both scopolamine and doenpezil were dissolved in saline solution. All reagents used in this study were of analytical grade and high purity.
Animals
Male Wistar rats (weighing 220-250 g, aged 8-10 months) obtained from the Animal House of the Institute were employed in the studies. The animals were kept in polyacrylic cages with wire mesh top and soft bedding. They were kept under standard husbandry conditions of 12h reverse light cycle with food and water ad libitum, maintained at temperature 22±2°C. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (RITS/IAEC/2013/01/01). Animals were acclimatized to laboratory conditions prior to experimentation.
Drug administration
EA was administered by oral (p.o.) route in dose of 25 mg/kg and 50 mg/kg. Scopolamine was administered by intraperitoneal (i.p.) route in dose of 0.7 mg/kg. Donepezil was administered in dose of 0.5 mg/kg, p.o.
Six groups (each group consist six rats) were employed in the present study. (i) Group1-Normal Control (ii) Group2-Scopolamine Control (0.7mg/kg,i.p.) (iii) Group3-EA Perse (50mg/kg, p.o.) 25mg/ kg, p.o. + Scopolamine (0.7mg/kg, i.p.) (vi) Group6-EA 50mg/kg, p.o. + Scopolamine (0.7mg/kg, i.p.). After a 5-day habituation period, rats were given EA (25 or 50 mg/kg, p.o.) and Donepezil (0.5 mg/kg, p.o.) for total of 13 days. EA or Donepezil alone was treated for 6 days and then scopolamine (0.7 mg/kg, i.p.) was administered together with EA for another 7 days. Rats underwent locomotor activity (LMA) for 2 days i.e. 6th day and 13th day, MWM test for 5 days i.e. 7th day to 11th day. The day after completion of Morris water maze (MWM), the elevated plus maze (EPM) was conducted for 2 days i.e. 12th to 13th day. The day after EPM, the rats were sacrificed and biochemical parameters were estimated (Figure 2).
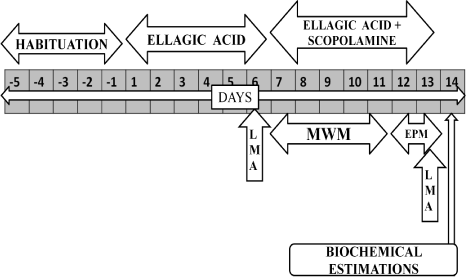
Figure 2: Protocol schedule to determine the neuroprotective effect of
Ellagic Acid in scopolamine induced Alzheimer’ type memory and cognitive
dysfunctions.
Elevated plus maze
Elevated plus maze (EPM) served as the behavioral model (where in the stimulus existed outside the body) to evaluate learning and memory in rats. It consists of two opened arms (50cm*10cm) and two covered arms (50cm*40cm*10cm). The arms were extended from central platform (10cm*10cm), and the maze was kept elevated to a height of 50cm from the floor. The EPM was conducted for 2 days i.e. 12th to 13th day of protocol schedule. Each animal was kept at the end of an open arm, facing away from the central platform on 12th day. Transfer Latency (TL), which was taken as the time taken by the animal to move into any one of the covered arms with all its four legs, recorded on 12th day i.e. acquisition trial [75]. If the rat did not enter into one of the covered arms within 120s then it was gently pushed into one of the two covered arms and the TL was assigned as 120s. The rats were allowed to explore the maze for 10s and then were returned to its home cage. TL was again examined 24hr after the first trial on 13th day of protocol schedule i.e. retention latency.
Spatial navigation task in Morris water maze
Morris water maze employed in the present study was a model to evaluate spatial learning and memory. Escape from water itself acts as motivation and eliminates the use of other motivational stimuli such as food and water deprivation. Water provides uniform environment and eliminates interference due to olfactory clues [76]. Animals were trained to swim to a platform in a circular pool (180cm diameter*60cm) located in a sound attenuated dark test room. The pool was filled with water (28±2°C) to a depth of 40cm. A movable circular platform, 9cm in diameter and mounted on a column, was placed in the pool 2 cm below the water level for escape latency time (ELT), while during time spent in the target quadrant (TSTQ) the platform was removed. Four equally spaced locations around the edge of the pool (N, S, E, and W) were used to divide the pool into 4 quadrants and one of them is used as start point, which was same during all trials. The pool was filled with opaque water to prevent visibility of the platform in the pool. The escape platform was placed in the middle of one of the random quadrants of the pool and kept in the same position throughout the experiments. Animals received a training session consisting of day 7 to 10 and ELT was recorded. ELT defined as the time taken by the animal to locate the hidden platform. ELT was noted as an index of learning. Each animal was subjected to single trial for four consecutive days (starting form 7th day of EA administration to 10th day), during which they were allowed to escape on the hidden platform and to remain there for 20 s. If the rats failed to find the platform within 120 s, it was guided gently onto the platform and allowed to remain there for 20 s.
On fifth day (i.e., 11th day of EA administration) the platform was removed. Rats were placed in water maze and allowed to explore the maze for 120 s. Time spent in three quadrants, that is, Q1, Q2 and Q3 was recorded and TSTQ in search of the missing platform provided as an index of retrieval. Care was taken not to disturb the relative location of water maze with respect to other objects in the laboratory.
Assessment of locomotor activity
Gross behavioral activity was assessed by digital actophotometer on 6th day and 13th day of protocol schedule to rule out any interference in locomotor activity by drugs which may affect the process of learning and memory, in before and after of MWM task. Each animal was observed over a period of 5 min in a square (30 cm) closed arena equipped with infrared light-sensitive photocells and values expressed as counts per 5 min [77]. The beams in the actophotometer, cut by the animal, were taken as measure of movements. The apparatus was placed in a darkened, sound-attenuated and ventilated testing room.
Preparation of brain homogenate
On 14th day of protocol schedule, Animals were sacrificed by decapitation, brains removed and rinsed with ice cold isotonic saline solution. Brain tissue samples were then homogenized with 10 times (w/v) ice cold 0.1M phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 x g for 15min, supernatant was separated and aliquots were used for biochemical estimations [77].
Protein estimation
On 14th day of protocol schedule, Animals were sacrificed by decapitation, brains removed and rinsed with ice cold isotonic saline solution. Brain tissue samples were then homogenized with 10 times (w/v) ice cold 0.1M phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 x g for 15min, supernatant was separated and aliquots were used for biochemical estimations [77].
Protein estimation
The protein content was measured by using Agappe protein estimation kit (Biuret method).
Estimation of Acetylcholinesterase levels
The quantitative measurement of AChE activity in brain was performed according to the method described by Ellman et al. (1961) [78]. The enzymatic activity in the supernatant was expressed as nmol per mg protein.
Estimation of malondialdehyde
The quantitative measurement of MDA– end product of lipid peroxidation-in brain homogenate was performed according to the method of Wills (1966) [79]. The concentration of MDA was expressed as nmol per mg protein.
Estimation of reduced glutathione
GSH in brain was estimated according to the method described by Ellman et al. (1959) [80]. The concentration of glutathione in the supernatant expressed as μmol per mg protein.
Estimation of superoxide dismutase activity
SOD activity was measured according to the method described by Misra and Frodvich (1972) [81]. The activity of SOD was expressed as % activity.
Estimation of Catalase activity
Catalase activity was measured by the method of Aebi (1974) [82]. The activity of catalase was expressed as % activity.
Statistical analysis
All the results and data were expressed as mean ± standard deviation. Data was analyzed using two way ANOVA followed by post hoc test Bonferroni and one way ANOVA followed by post hoc test Tukey’s multi-comparison test. P<0.05 was considered as statistically significant.
Results
Effect of Ellagic acid on rats in elevated plus maze
On 12th day of protocol schedule, acquisition latency was recorded. Retention was observed as transfer latency (TL) on 13th day to evaluate learning and memory in rats using EPM. On 12th and 13th day Scopolamine administered rats showed remarkable increase (p<0.001) (113±9.3 and 106.5±11.1 sec) in TL, when compared to normal (64±4.2 and 36.833±6.7 sec) and EA perse rats (63.333±10.3 and 32.833±3.3 sec). During experiment, EA perse administration did not reveal any change (p>0.05), when compared to normal rats in TL. Donepezil, a well established standard drug for AD considerably decrease (p<0.001) (65.5±13.0 and 21.666±5.0 sec) TL, when compared to Scopolamine administered rats and reversed the memory impairment induced by Scopolamine. Administration of EA at the dose of 25 mg/kg, p.o. exhibit notable decrease (p<0.001) (72.00±8.0 and 39.333±6.1 sec) in TL, when compared to Scopolamine treated rats. EA (50 mg/kg, p.o.) administration also decrease (p<0.001) (69.333±8.0 and 25.333±3.8 sec) TL, when compared to Scopolamine administered rats and there were significant variation (p<0.05) was found in between doses of EA 25 & 50 mg/kg, p.o. indicating improved retention memory. Donepezil administered rats did not reveal any change (p>0.05) in TL, when compared to EA (25 or 50 mg/kg, p.o.) administered rats (Figure 3).
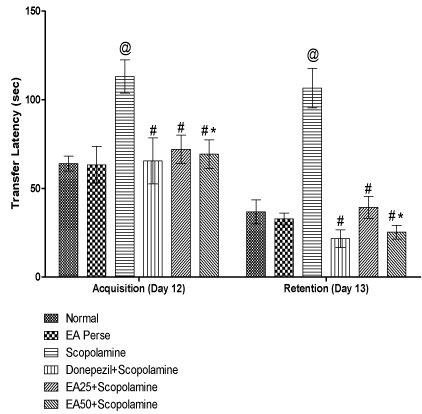
Figure 3: Effect of Ellagic acid on Transfer latency of rats using Elevated
Plus Maze.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Effect of Ellagic acid on rats in spatial navigation task using Morris water maze
On 7th to 10th day of 14 day protocol schedule, escape latency time (ELT) was observed. On 7th day, there were no significant changes (p>0.05) observed in Scopolamine (94.33±13.1 sec) treated rats, when compared to normal (89±9.8 sec) and EA perse treated (86.33±13.9 sec) rats. EA perse administration did not show any significant change (p>0.05) when compared to normal rats. Moreover, Donepezil treated rats did not show any considerable changes (p>0.05) (88±9.0 sec), when compared to Scopolamine and EA (25 or 50 mg/kg, p.o.) administered rats. In the treatment groups, administration of EA did not confirm notable changes (p>0.05) (96.33±10.0; 88.66±10.6 sec) in ELT at 25 and 50 mg/kg, p.o. when compared to Scopolamine treated rats. There were no changes (p>0.05) found in ELT between treatment doses of EA 25 & 50 mg/kg, p.o.
Comparison data of 8th day, 9th day and 10th day ELT in MWM, showed that Scopolamine administered rats manifest remarkable increase (p<0.05, p<0.001 and p<0.001) (92±8.1, 85.33±12.7 and 83.33±8.6 sec) in ELT, when compared to normal (76.33±7.8, 29.16±7.8 and 15.33±3.7 sec) and EA perse (67.33±5.6, 29.33±8.7 and 15±2.8 sec) rats. EA perse administration did not show any significant difference (p>0.05), when compared to normal rats during ELT. Donepezil administered rats significantly decreased (p<0.001, p<0.001 and p<0.001) (51±10.1, 26.16±6.4 and 10.83±4.6 sec) ELT when compared to Scopolamine administered rats. EA at 25 mg/kg, p.o. proved remarkable decreased (p>0.05, p<0.001 and p<0.001) (79±10.8, 60.83±8.6 and 38.16±9.7 sec) in the ELT, when compared to Scopolamine administered rats. EA at the dose 50 mg/kg, p.o. significantly decreased (65.33±11.7, 43±9.8 and 24.5±8.3 sec) the ELT, when compared to Scopolamine (p<0.001, p<0.001 and p<0.001) and EA 25 mg/kg, p.o. treated rats (p<0.05, p<0.01 and p<0.05), indicating remarkable improvement in learning. Donepezil administered rats more significantly decreased ELT when compared to EA 25 mg/kg, p.o. (p<0.001, p<0.001 and p<0.001) and 50 mg/kg, p.o. administered rats (p<0.005, p<0.001 and p<0.005) (Figure 4).
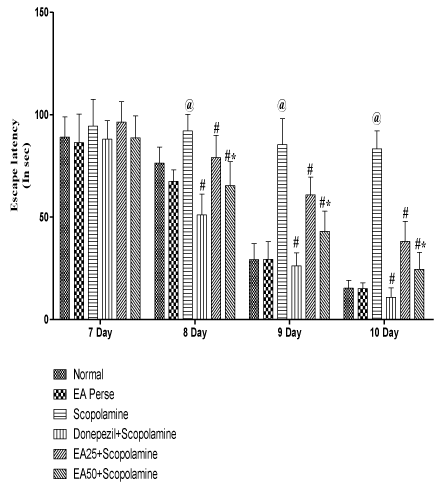
Figure 4: Effect of Ellagic acid on Escape latency time of rats on 7th day to
10th day using Morris Water Maze.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
On 11th day of protocol schedule TSTQ was performed. Time spent in target quadrant (TSTQ) in search of missing platform provided as an index of retrieval. Scopolamine treated rats showed remarkable decrease (p<0.001) (7.667±3.0 sec) in TSTQ when compared to normal (45.17±8.0 sec) and EA perse treated (43.83±6.2 sec) rats. In perse group of EA, there were no changes (p>0.05) during TSTQ when compared to normal group. Further, Donepezil administered rats improved (p<0.001) (46.17±5.3 sec) memory when compared to Scopolamine treated rats. EA (25 mg/kg, p.o.) administration showed remarkable increase (p<0.05) (19.50±1.517 sec) in TSTQ when compared to Scopolamine treated rats. EA (50 mg/kg, p.o.) administration indicated improvement (p<0.001) (32.00±8.1 sec) in memory function when compared with Scopolamine administered rats. Moreover, markedly difference (p<0.05) was also observed in between treatment doses of EA. Donepezil administered rats showed more significant improved memory when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.05) (Figure 5).

Figure 5: Effect of Ellagic acid on time spent in target quadrant of rats using
Morris Water Maze.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine.
Effect of Ellagic acid on rats in locomotor activity
On 6th day and 13th day of protocol schedule, locomotor activity was observed to rule out any interference in locomotion by treatment drug. Scopolamine employed rats did not reveal any significant changes (p>0.05) (281.333±15.3 and 274.833±5.3) in locomotor activity when compared to normal (263.833±17.4 and 274.5±21.3) and EA perse (270.666±18.2 and 274.5±4.7) rats. EA perse administration also did not show any considerable change (p>0.05) in locomotor activity at 50 mg/kg, p.o. when compared to normal rats. Donepezil treated also showed insignificant changes (p>0.05) (267.5±21.3 and 274.833±5.3) when compared to Scopolamine and EA (25 or 50 mg/kg, p.o.) treated rats. EA 25 mg/kg, p.o. (266.833±15.4 and 270.833±20.6) and 50 mg/kg, p.o. (274.5±4.764 and 283.5±16.208) administration did not showed any notable changes (p>0.05) in locomotor activity of rats when compared to Scopolamine treated rats, indicating there were no effect on locomotor activity (Figure 6).

Figure 6: Effect of Ellagic acid on locomotor activity of rats using
actophotometer.
Values were mean ± SD
Effect of Ellagic acid on acetylcholinesterase levels
Prolongation of availability of acetylcholine has been used to enhancing cholinergic function. This prolongation may be achieved by inhibiting AChE. Scopolamine administered rats significantly increased (p<0.001) (415.0±19.6) the AChE level when compared to normal (136.8±4.9) and EA perse (137.2±4.1) rats. EA perse administration did not show any appreciable changes (p>0.05) in AChE level at the dose of 50 mg/kg, p.o. when compared to normal rats. Donepezil treated rats appreciably decreased (p<0.001) (231.0±7.6) the AChE level in contrast to Scopolamine administered rats. EA (25 mg/kg, p.o.) showed remarkably diminished (p<0.001) the AChE level (360.8±15.9) when compared to Scopolamine rats. Administration of EA (50 mg/kg, p.o.) significantly reduced (p<0.001) (311.7±17.6) the AChE level when compared to Scopolamine administered rats. Moreover, there were expressive distinction (p<0.001) was present in between treatment doses of EA. In Donepezil administered rats, AChE level was more significantly decreased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.01) (Figure 7).
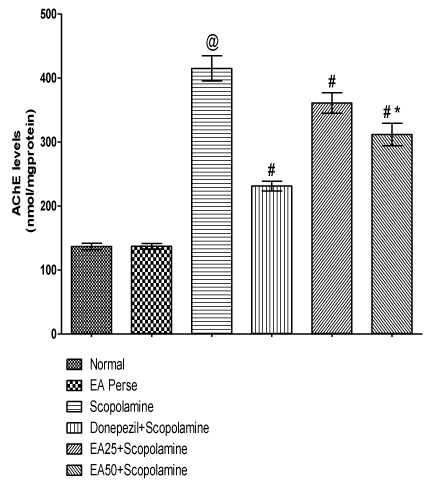
Figure 7: Effect of Ellagic acid on Acetylcholinesterase levels.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Effect of Ellagic acid on malondialdehyde levels
On 6th day and 13th day of protocol schedule, locomotor activity was observed to rule out any interference in locomotion by treatment drug. Scopolamine employed rats did not reveal any significant changes (p>0.05) (281.333±15.3 and 274.833±5.3) in locomotor activity when compared to normal (263.833±17.4 and 274.5±21.3) and EA perse (270.666±18.2 and 274.5±4.7) rats. EA perse administration also did not show any considerable change (p>0.05) in locomotor activity at 50 mg/kg, p.o. when compared to normal rats. Donepezil treated also showed insignificant changes (p>0.05) (267.5±21.3 and 274.833±5.3) when compared to Scopolamine and EA (25 or 50 mg/kg, p.o.) treated rats. EA 25 mg/kg, p.o. (266.833±15.4 and 270.833±20.6) and 50 mg/kg, p.o. (274.5±4.764 and 283.5±16.208) administration did not showed any notable changes (p>0.05) in locomotor activity of rats when compared to Scopolamine treated rats, indicating there were no effect on locomotor activity (Figure 6).
Effect of Ellagic acid on acetylcholinesterase levels
Prolongation of availability of acetylcholine has been used to enhancing cholinergic function. This prolongation may be achieved by inhibiting AChE. Scopolamine administered rats significantly increased (p<0.001) (415.0±19.6) the AChE level when compared to normal (136.8±4.9) and EA perse (137.2±4.1) rats. EA perse administration did not show any appreciable changes (p>0.05) in AChE level at the dose of 50 mg/kg, p.o. when compared to normal rats. Donepezil treated rats appreciably decreased (p<0.001) (231.0±7.6) the AChE level in contrast to Scopolamine administered rats. EA (25 mg/kg, p.o.) showed remarkably diminished (p<0.001) the AChE level (360.8±15.9) when compared to Scopolamine rats. Administration of EA (50 mg/kg, p.o.) significantly reduced (p<0.001) (311.7±17.6) the AChE level when compared to Scopolamine administered rats. Moreover, there were expressive distinction (p<0.001) was present in between treatment doses of EA. In Donepezil administered rats, AChE level was more significantly decreased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.01) (Figure 7).
Effect of Ellagic acid on malondialdehyde levels
MDA is an indicator of lipid peroxidation. Scopolamine administration increased (p<0.001) (42.50±3.0) the MDA level when compared to normal (19.88±0.9) and EA perse (19.15±1.8) rats. Further, EA perse administration did not show any considerable changes (p>0.05) in MDA levels when compared to normal rats. Donepezil appreciably decreased (p<0.001) (23.12±0.5) the MDA level when compared to Scopolamine administered rats. EA (25 mg/kg, p.o.) administration showed remarkably decrease (p<0.001) (33.57±3.3) in MDA level when compared to Scopolamine administered rats. EA administered rats at the dose of 50 mg/kg, p.o significantly decreased (27.97±2.0) in MDA level when compared to Scopolamine (p<0.001) and EA 25 mg/kg, p.o. treated rats (p<0.05). In Donepezil administered rats, MDA level was more significantly decreased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/ kg, p.o. administered rats (p<0.05) (Figure 8).
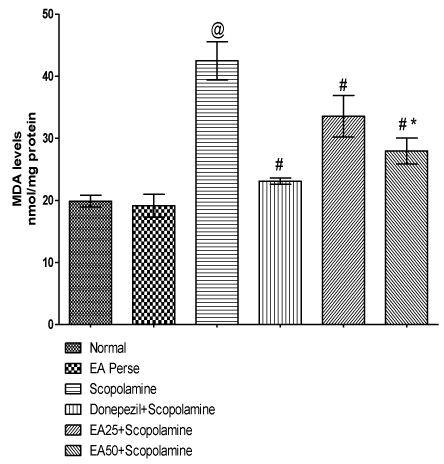
Figure 8: Effect of Ellagic acid on Malondialdehyde levels.
Values were mean ± SD, @ p<0.05 as compared to normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Effect of Ellagic acid on reduced glutathione levels
Reduced GSH is a marker of cellular antioxidant and provide protection against oxidative stress. Scopolamine governed rats remarkably decreased (p<0.001) (2.067±0.4) the GSH level when compared to normal (9.833±0.7) and EA perse treated (9.733±0.7) rats. EA perse administration did not show any considerable changes (p>0.05) in GSH levels in contrast to normal rats. Donepezil significantly increase (p<0.001) (7.767±0.3) the GSH levels when compared to Scopolamine treated rats. EA (25 mg/kg, p.o.) administration exhibited remarkable increase (p<0.001) (5.250±0.5) in GSH level when compared to Scopolamine treated rats. EA (50 mg/kg, p.o.) showed significantly increase (p<0.001) (6.317±0.3) in GSH level when compared to Scopolamine treated rats. Moreover, in between treatment doses of EA, there were significance difference (p<0.05) was present. In Donepezil administered rats, GSH level was more significantly increased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.05) (Figure 9).

Figure 9: Effect of Ellagic acid on reduced Glutathione levels.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Effect of Ellagic acid on superoxide dismutase activity
SOD is an antioxidant enzyme, which plays a key role in detoxifying superoxide anions. Scopolamine administered rats significantly decreased (p<0.001) (27.33±3.3) the SOD levels in brain homogenate when compared to normal (100.0±0.0) and EA perse (95.83±2.6) rats. EA perse administration did not reveal any considerable change (p>0.05) in SOD activity when compared to normal rats. Donepezil expressively increase (p<0.001) (82.00±3.9) SOD activity when compared to Scopolamine treated rats. In treatment group, EA (25 mg/kg, p.o.) administration showed remarkable increase (p<0.001) (59.17±8.0) in SOD activity when compared to Scopolamine treated rats. EA (50 mg/kg, p.o.) administration showed significantly increase (p<0.001) (71.33±4.0) in SOD activity when compared to Scopolamine treated rats and there were remarkably disparity (p<0.001) was found in between EA treated groups. In Donepezil administered rats, SOD activity was more significantly increased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.05) (Figure 10).
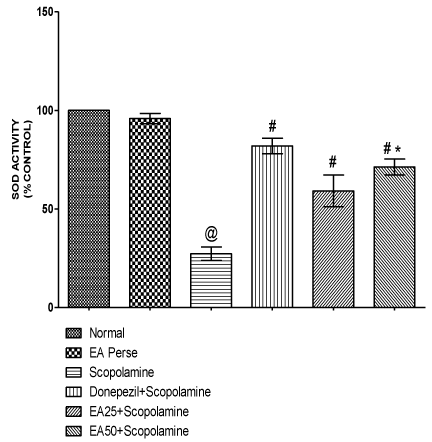
Figure 10: Effect of Ellagic acid on Superoxide dismutase activity.
Values were mean ± SD, @ p<0.05 as compared to Normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Effect of Ellagic acid on catalase activity
Catalase is also an antioxidant enzyme which has capability to detoxify oxidative free radicals. Scopolamine treated rats manifested remarkable decrease (p<0.001) (36.50±4.4) in catalase activity in brain homogenate when compared to normal (100.0±0.0) and EA perse treated (95.50±1.8) rats. EA perse administration did not show any considerable changes (p>0.05) in catalase activity when compared to normal rats. Donepezil significantly increase (p<0.001) (81.67±4.0) in catalase activity when compared to Scopolamine treated (36.50±4.4) rats. EA (25 mg/kg, p.o.) remarkably increased (p<0.001) (59.17±4.5) the catalase activity when compared to Scopolamine treated rats. EA (50 mg/kg, p.o.) administration exhibited significantly increase (p<0.001) (73.67±3.5) in catalase activity when compared to Scopolamine and EA 25 mg/kg, p.o. treated rats. In Donepezil administered rats, catalase activity was more significantly increased when compared to EA 25 mg/kg, p.o. (p<0.01) and 50 mg/kg, p.o. administered rats (p<0.05) (Figure 11).
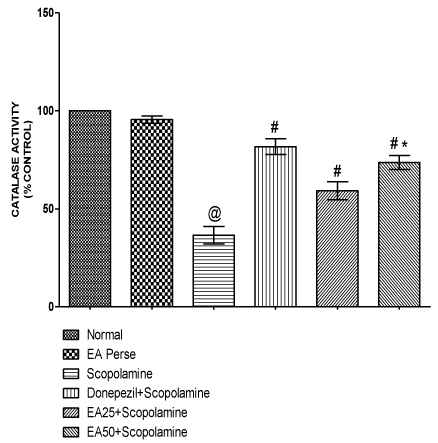
Figure 11: Effect of Ellagic acid on catalase activity.
Values were mean ± SD, @ p<0.05 as compared to normal & EA perse,
# p<0.05 as compared to Scopolamine, * p<0.05 as compared to EA 25 +
Scopolamine
Discussion
Clinically AD is characterized by an insidious degradation of memory, associated with functional decline and neurobehavioral disturbances [83]. Despite the availability of various treatment strategies, the severity and prevalence of this disease are not yet under control. Therefore, alternative and complementary medicines including herbal supplements, phytochemicals and extracts are being utilized in the management of AD [84-87]. The current hypothesis about the mechanisms by which neurons come into necrotic or apoptotic processes has led to believe that the therapeutic use of natural antioxidants may be beneficial in aging and neurodegenerative disorders [88,89].
In the present study, the effect of improving memory deficit of EA was evaluated using scopolamine induced Alzheimer’s type dementia in rats.
Scopolamine induced Alzheimer’s type dementia model has been widely used to provide a pharmacological model of memory dysfunction for screening potential cognition enhancing agents [47,55]. The cognitive-enhancing activity of EA on scopolamine induced memory impairments in rats was investigated by using behavioral and biochemical parameters.
During elevated plus maze, decrease in retention latency indicated improvement of memory and vice versa [90]. In EPM, it was shown that long term injected scopolamine also drastically increase in TL, demonstrating that the central cholinergic neuronal system plays an important role in learning acquisition. EA dose-dependently decreased TL prolongation induced by scopolamine. These results suggested that the neuroprotective effect of EA on scopolamineinduced memory impairment may be related to mediation of the cholinergic nervous system.
In order to confirm the effects of EA, MWM was used to test spatial learning in rats, where scopolamine treated rats were taking more time to reach at the hidden platform which shows memory impairments in this spatial task. EA treated rats impressively reduced the escape latency prolonged by scopolamine. Moreover, EA exhibited appreciable improvement of cognitive performance as indicated by significant decrease in ELT. It is important to notice that MWM test investigating spatial learning and memory has been used in detecting changes of the central cholinergic system [91,92]. If the animals spent more time in target quadrant where the platform had previously been placed during the training session, this would indicate that the animals learned from prior experience with the MWM test, showing the spatial memory improvement. Scopolamine treated rats decreased TSTQ, on the other side EA treated rats expressively increased the TSTQ. Both the test doses viz., 25 mg/kg, p.o. and 50mg/ kg, p.o. significantly attenuated these behavioral changes in rats with scopolamine induced memory and cognitive impairment.
Along with EPM and MWM, Locomotor activity also was investigated using actophotometer to determine any modulation in locomotor activity by treatment drugs which may affect locomotion in EPM and MWM. However no significant difference in locomotor activity was observed in any of the animal groups. These results suggest that there was not any sedative effect or interference in EPM and MWM locomotion. Therefore, transfer latency in EPM, escape latency and TSTQ in MWM were purely result of improved memory. Therefore, EA can repair the long-term memory in scopolamineinduced memory impairments.
To investigate the effect of EA on cholinergic function, that governs vital aspects of memory and other cognitive functions, brain acetylcholinesterase activity was measured in the present study. The hippocampus, amygdala and cortical regions of the brain are mainly involved in cholinergic transmission to monitor learning and memory processing and seem to be more prone to oxidative damage [93].
In this study, scopolamine was found to significantly elevate AChE activity, an enzyme responsible for degradation of Ach, which is in tune with earlier reports [58]. This increase in AChE activity was significantly restored dose dependently by EA. These observations suggest the modulation of cholinergic neurotransmission and/or prevention of cholinergic neuronal loss.
Recently, many studies have reported that memory impairments is associated to oxidative damage in the scopolamine-induced dementia in rats [55].
Lipid peroxidation is an important indicator of neurodegeneration of brain. Unlike other body membranes, neuronal membranes contain a very high percentage of long chain polyunsaturated fatty acids because they are used to construct complex structures needed for high rates of signal transfer. ROS are generated continuously in nervous tissues during normal metabolism and neuronal activity. The brain is subjected to free radical induced lipid peroxidation because it uses one-third of the inspired oxygen [94,95]. Lipids and proteins, the major structural and functional components of the cell membrane, are the target of oxidative modification by free radicals in neurodegenerative disorders [96]. Extensive evidence exists on lipid peroxidation and protein oxidation leading to loss of membrane integrity, an important factor in acceleration of aging and age-related neurodegenerative disorders. Oxidative stress has been implicated in the pathogenesis of AD in humans [97,98].
In the present study, scopolamine-injection in rats significantly induced peroxidation of lipids and proteins, and reduced antioxidant defense indicating increased oxidative stress. MDA is an end product of lipid peroxidation and is a measure of free radical generation and scopolamine injected rats showed extensive lipid peroxidation as evidenced by increase in MDA levels. In order to evaluate the effect of EA on lipid peroxidation in brain, MDA level was assessed. MDA level was remarkably increased by scopolamine and EA dosedependently reduced MDA level, indicating the reduced peroxidation of lipids.
Lipid peroxidation may enhance due to depletion of GSH content in the brain, which is often considered as the first line of defense of the cell by this endogenous antioxidant against oxidative stress [96,99]. Evidence has been presented that the neuronal defense against H2O2, which is the most toxic molecule to the brain, is mediated primarily by the glutathione system [100,101]. GSH is a tri-peptide, an endogenous antioxidant found in all animal cells in variable amounts and is a very accurate indicator of oxidative stress [102]. Consistent with previous studies, in present study, scopolamine treatment significantly decreased the GSH levels. Further, co-administration of EA markedly improved GSH levels.
The most important antioxidant enzymes are SOD and catalase. SOD plays a key role in detoxifying superoxide anions, which otherwise damages the cell membranes and macromolecules. Scopolamine administration showed a significant reduction in enzymatic activity of SOD and catalase. On the other side, Catalase has the capability to detoxify H2O2 radicals. Release of H2O2 promotes the formation of numerous other oxidant species that greatly contributes for oxidative stress leading to the pathogenesis of AD [103]. Scopolamine treatment was found to be decreased SOD and catalase activities. Treatment of rats with EA significantly preserved the activities of SOD and catalase.
The results of the present study suggest that chronic administration of EA perse did not have any significant effect on cognitive performance in normal animals. But, EA treatment groups at the dose of 25 & 50 mg/kg, p.o. showed marked improvement in cognitive tasks when compared to scopolamine treated rats suggesting the significant role of ACh in long lasting administrated scopolamine mediated cognitive dysfunction. Reports also support that ACh is involved in memory acquisition and retention [104,105]. Moreover, scopolamine injection drastically impaired memory retention, resembling Alzheimer’s dementia [50,55]. The same has been reported to be attenuated by pretreatment with herbal supplements and extracts, and phytochemicals [85,86].
The presented data in this study also suggests that EA possesses potent antioxidant activity by scavenging ROS and exerting a neuroprotective effect against oxidative damage induced by scopolamine. Predominant role of AChE inhibition, antioxidant activity reveal an important contributory factor to the beneficial effects of EA against dementia. Higher dose of Ellagic acid i.e. 50 mg/kg, p.o. was found more neuroprotective in all behavioral and biochemical evaluations. At lastly, the neuroprotective effects of EA might result from the regulation of AChE and the anti-oxidative defense system. These results suggest that EA can be used as a preventive herbal drug to impede cholinergic dysfunctions and oxidative stress in AD.
Conclusion
It was concluded that administration of scopolamine could cause Alzheimer’s type dementia via increase AChE levels and oxidative stress. Scopolamine mediated Alzheimer’s type dementia is mainly associated with cognitive and memory impairments in behavioral models. On the basis of this study, the major bio-markers of Alzheimer’s disease like amyloid beta, inflammatory cytokines and histopathological changes can be further evaluated according to current protocol schedule to confirm and justify the strong evidence of Ellagic acid in long term injected scopolamine mediated dementia. Ellagic acid diminished the acetylcholinesterase level and improves the anti-oxidant defense system. Further, Ellagic acid downturned the cognitive impairments induced by scopolamine. Like Donepezil, Ellagic acid reversed the scopolamine induced Alzheimer’s type dementia in rats.
Acknowledgement
We express our gratitude to Chairman Dr. Rajendra Singh, Secretary Dr. Om Parkash Rajendra Institute of Technology and Sciences, Sirsa, Haryana, India for their inspiration and constant support. We also great thankful to Assistant professor Mr. Raghuvir Singh and Mr. Mohit Mehta, Department of Pharmaceutical Sciences, RITS, Sirsa for their instant contributions and services.
References
- Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014; 76 Pt A: 27-50.
- Jellinger KA. Alzheimer 100--highlights in the history of Alzheimer research. J Neural Transm. 2006; 113: 1603-1623.
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002; 287: 3223-3229.
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary Intake of Antioxidant Nutrients and the Risk of Incident Alzheimer Disease in a Biracial Community Study. JAMA. 2002; 287: 3230-3237.
- Staehelin HB. Neuronal protection by bioactive nutrients. Int J Vitam Nutr Res. 2008; 78: 282-285.
- Harvey BS, Musgrave IF, Ohlsson KS, Fransson A, Smid SD. The green tea polyphenol (-)-epigallocatechin-3-gallate inhibits amyloid-β evoked fibril formation and neuronal cell death in vitro. Food Chemistry. 2011; 129: 1729-1736.
- Bastianetto S, Ramassamy C, Doré S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci. 2000; 12: 1882-1890.
- Craggs L, Kalaria RN. Revisiting dietary antioxidants, neurodegeneration and dementia. Neuroreport. 2011; 22: 1-3.
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001; 21: 8370-8377.
- Gomez-Pinilla F, Nguyen TT. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr Neurosci. 2012; 15: 127-133.
- Anhe FF, Desjardins Y, Pilon G, Dudonne S, Genovese M, Lajolo FM, et al. Polyphenols and type 2 diabetes: A prospective review. Pharma Nutrition. 2013; 1: 105–114.
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006; 24: 506-515.
- Nantitanon W, Yotsawimonwat S, Okonogi S. Factors influencing antioxidant activities and total phenolic content of guava leaf extract. LWT - Food Sci Technol. 2010; 43: 1095-1103.
- Landete JM. Ellagitannins, ellagic acid and their derived metabolites: A review about source metabolism, functions and health. Food Res Int. 2011; 44: 1150–1160.
- Yüce A, Ateşşahin A, Ceribaşi AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007; 101: 345-349.
- Özkaya A, Celik S, Yüce A, Sahin Z, Yilmaz O. The Effects of Ellagic Acid on Some Biochemical Parameters in the Liver of Rats against Oxidative Stress Induced by Aluminum. Kafkas Univ Vet Fak DerG. 2010; 16: 263-268.
- Türk G, Sönmez M, Ceribaşi AO, Yüce A, Ateşşahin A. Attenuation of cyclosporine A-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int Immunopharmacol. 2010; 10: 177-182.
- Umesalma S, Sudhandiran G. Differential Inhibitory Effects of the Polyphenol Ellagic Acid on Inflammatory Mediators NF-jB, iNOS, COX-2, TNF-a, and IL-6 in 1,2-Dimethylhydrazine-Induced Rat Colon Carcinogenesis. Basic Clin Pharmacol Toxicol. 2010; 107: 650-655.
- Rosillo MA, Sánchez-Hidalgo M, Cárdeno A, Aparicio-Soto M, Sánchez-Fidalgo S, Villegas I, et al. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res. 2012; 66: 235-242.
- Malik A, Afaq S, Shahid M, Akhtar K, Assiri A. Influence of ellagic acid on prostate cancer cell proliferation: a caspase-dependent pathway. Asian Pac J Trop Med. 2011; 4: 550-555.
- Qiu Z, Zhou B, Jin L, Yu H, Liu L, Liu Y, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol. 2013; 59: 428-437.
- Zhao M, Tang SN, Marsh JL, Shankar S, Srivastava RK. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Lett. 2013; 337: 210-217.
- Malini P, Kanchana G, Rajadurai M. Antidiabetic efficacy of ellagic acid in streptozotocin induced diabetes mellitus in albino wistar rats. Asian J Pharm Clin Res. 2011; 4: 124-128.
- You Q, Chen F, Wang X, Jiang Y, Lin S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT - Food Sci Technol. 2012; 46: 164-168.
- Kannan MM, Quine SD. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism. 2013; 62: 52-61.
- Rani UP, Kesavan R, Ganugula R, Avaneesh T, Kumar UP, Reddy GB, et al. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem. 2013; 24: 1830-1839.
- Shukitt-Hale B, Lau FC, Carey AN, Galli RL, Spangler EL, Ingram DK, et al. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci. 2008; 11: 172-182.
- Uzar E, Alp H, Cevik MU, Firat U, Evliyaoglu O, Tufek A, et al. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012; 33: 567-574.
- Muthaiyah B, Essa MM, Chauhan V, Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011; 36: 2096-2103.
- Gaire BP, Jamarkattel-Pandit N, Lee D, Song J, Kim JY, Park J, et al. Terminalia chebula extract protects OGD-R induced PC12 cell death and inhibits LPS induced microglia activation. Molecules. 2013; 18: 3529-3542.
- Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated t-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J Nutr. 2013; 143: 597-605.
- Wilson GN, Mickley GA, Matera KM. The efficacy of ellagic acid in attenuating neurophysiological and cognitive-behavioral symptoms associated with infusion of amyloid-beta (Aß) peptide fragments in adult rats. The Baldwin-Wallace College Journal of Research and Creative Studies, Spring. 2010; 3: 15-30.
- Sheean P, Rout MK, Head RJ, Bennett LE. Modulation of in vitro activity of zymogenic and mature recombinant human ß-secretase by dietary plants. FEBS J. 2012; 279: 1291-1305.
- Messier C, Gagnon M. Glucose regulation and cognitive functions: relation to Alzheimer's disease and diabetes. Behav Brain Res. 1996; 75: 1-11.
- Poulose N, Prasad CNV, Haridas PAN, Anilkumar G. Ellagic acid stimulates glucose transport in adipocytes and muscles through AMPK mediated pathway. J Diabetes Metab. 2011; 2: 7.
- Makino-Wakagi Y, Yoshimura Y, Uzawa Y, Zaima N, Moriyama T, Kawamura Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem Biophys Res Commun. 2012; 417: 880-885.
- Girish C, Raj V, Arya J, Balakrishnan S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur J Pharmacol. 2013; 710: 49-58.
- Girish C, Raj V, Arya J, Balakrishnan S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur J Pharmacol. 2012; 682: 118-125.
- Tatton W, Chen D, Chalmers-Redman R, Wheeler L, Nixon R, Tatton N. Hypothesis for a common basis for neuroprotection in glaucoma and Alzheimer's disease: anti-apoptosis by alpha-2-adrenergic receptor activation. Surv Ophthalmol. 2003; 48: S25-37.
- Wenk GL, McGann K, Hauss-Wegrzyniak B, Rosi S. The toxicity of tumor necrosis factor-alpha upon cholinergic neurons within the nucleus basalis and the role of norepinephrine in the regulation of inflammation: implications for Alzheimer's disease. Neuroscience. 2003; 121: 719-729.
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005; 102: 15653-15658.
- Madsen K, Neumann WJ, Holst K, Marner L, Haahr MT, Lehel S, et al. Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer's disease. J Alzheimers Dis. 2011; 26: 457-466.
- Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther. 2013; 5: 21.
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001; 50: 2792-2808.
- Cheng X, Wu J, Geng M, Xiong J. Role of synaptic activity in the regulation of amyloid beta levels in Alzheimer's disease. Neurobiol Aging. 2014; 35: 1217-1232.
- Spencer DG, La H. Effects of Anticholinergic Drugs on Learning and Memory. Drug Develop. Res. 1983; 3: 489-502.
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004; 20: 1081-1088.
- Terry AV Jr. Muscarinic Receptor Antagonists in Rats. Muscarinic Receptor Antagonists in Rats. 2006.
- Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010; 649: 210-217.
- Kwon SH, Ma SX, Joo HJ, Lee SY, Jang CG. Inhibitory Effects of Eucommia ulmoides Oliv. Bark on Scopolamine-Induced Learning and Memory Deficits in Mice. Biomol Ther (Seoul). 2013; 21: 462-469.
- Tsukada H, Kakiuchi T, Ando I, Ouchi Y. Functional activation of cerebral blood flow abolished by scopolamine is reversed by cognitive enhancers associated with cholinesterase inhibition: a positron emission tomography study in unanesthetized monkeys. J Pharmacol Exp Ther. 1997; 281: 1408-1414.
- Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012; 232: 66-76.
- Hebbel RP, Shalev O, Foker W, Rank BH. Inhibition of erythrocyte Ca2+-ATPase by activated oxygen through thiol- and lipid-dependent mechanisms. Biochim Biophys Acta. 1986; 862: 8-16.
- El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary Eel-D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav. 2003; 76: 525-533.
- Hancianu M, Cioanca O, Mihasan M, Hritcu L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine. 2013; 20: 446-452.
- Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002; 133: 369-376.
- Jang YJ, Kim J, Shim J, Kim CY, Jang JH, Lee KW, et al. Decaffeinated coffee prevents scopolamine-induced memory impairment in rats. Behav Brain Res. 2013; 245: 113-119.
- Ahmad A, Ramasamy K, Jaafar SM, Majeed AB, Mani V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem Toxicol. 2014; 65: 120-128.
- Haroutunian V, Greig N, Pei XF, Utsuki T, Gluck R, Acevedo LD, et al. Pharmacological modulation of Alzheimer's beta-amyloid precursor protein levels in the CSF of rats with forebrain cholinergic system lesions. Brain Res Mol Brain Res. 1997; 46: 161-168.
- Bihaqi SW, Singh AP, Tiwari M. Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and Amyloid precursor protein (AßPP) expression in rat brain. Indian J Pharmacol. 2012; 44: 593-598.
- Preston GC, Brazell C, Ward C, Broks P, Traub M, Stahl SM. The scopolamine model of dementia: determination of central cholinomimetic effects of physostigmine on cognition and biochemical markers in man. J Psychopharmacol. 1988; 2: 67-79.
- Wesnes K, Anand R, Lorscheid T. Potential of moclobemide to improve cerebral insufficiency identified using a scopolamine model of aging and dementia. Acta Psychiatr Scand Suppl. 1990; 360: 71-72.
- Molchan SE, Mellow AM, Lawlor BA, Weingartner HJ, Cohen RM, Cohen MR, et al. TRH attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl). 1990; 100: 84-89.
- Lines CR, Ambrose JH, Heald A, Traub M. A double-blind, placebo-controlled study of the effects of eptastigmine on scopolamine-induced cognitive deficits in healthy male subjects. Human Psychopharmacology: Clinical and Experimental. 1993; 8: 271-278.
- Gattu M, Boss KL, Terry AV Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997; 57: 793-799.
- Buccafusco JJ. The Revival of Scopolamine Reversal for the Assessment of Cognition-Enhancing Drugs. The Revival of Scopolamine Reversal for the Assessment of Cognition-Enhancing Drugs. 2009.
- Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem. 2000; 7: 303-339.
- Bartolini M, Bertucci C, Cavrini V, Andrisano V. beta-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem Pharmacol. 2003; 65: 407-416.
- Kimura M, Akasofu S, Ogura H, Sawada K. Protective effect of donepezil against Abeta(1-40) neurotoxicity in rat septal neurons. Brain Res. 2005; 1047: 72-84.
- Reale M, Iarlori C, Gambi F, Feliciani C, Isabella L, Gambi D. The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer's disease patients. Neuropharmacology. 2006; 50: 606-613.
- Riedel G, Kang SH, Choi DY, Platt B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res. 2009; 204: 217-225.
- Lindner MD, Hogan JB, Hodges DB Jr, Orie AF, Chen P, Corsa JA, et al. Donepezil primarily attenuates scopolamine-induced deficits in psychomotor function, with moderate effects on simple conditioning and attention, and small effects on working memory and spatial mapping. Psychopharmacology (Berl). 2006; 188: 629-640.
- Snyder PJ, Bednar MM, Cromer JR, Maruff P. Reversal of scopolamine-induced deficits with a single dose of donepezil, an acetylcholinesterase inhibitor. Alzheimers Dement. 2005; 1: 126-135.
- Sumanth M, Sowmya H, Nagaraj SV, Narasimharaju K. Efficacy of donepezil and galantamine in retrograde amnesia. AJPCR. 2010; 3: 23-25.
- Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001; 68: 1021-1029.
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology. 2008; 33: 973-980.
- Kumar A, Dogra S, Prakash A. Neuroprotective Effects of Centella asiatica against Intracerebroventricular Colchicine-Induced Cognitive Impairment and Oxidative Stress. Int J Alzheimers Dis. 2009; 2009.
- Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961; 7: 88-95.
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966; 99: 667-676.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70-77.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247: 3170-3175.
- Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974; 48: 137-145.
- Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer's disease. Neurotherapeutics. 2008; 5: 433-442.
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000; 54: 2261-2268.
- Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L: chemistry, efficacy, safety, and uses. J Food Sci. 2008; 73: R14-19.
- Hajiaghaee R, Akhondzadeh S. Herbal Medicine in the Treatment of Alzheimer’s disease. J Med Plants. 2012; 11: 2-7.
- Downey LA, Kean J, Nemeh F, Lau A, Poll A, Gregory R, et al. An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother Res. 2013; 27: 1407-1413.
- Di Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord. 2003; 2: 95-107.
- McGhie TK, Walton MC, Barnett LE, Vather R, Martin H, Au J, et al. Boysenberry and blackcurrant drinks increased the plasma antioxidant capacity in an elderly population but had little effect on other markers of oxidative stress. J Sci Food Agri. 2007; 87: 2519-2527.
- Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl). 1990; 101: 27-33.
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001; 36: 60-90.
- Hosseini-Sharifabad A, Mohammadi-Eraghi S, Tabrizian K, Soodi M, Khorshidahmad T, Naghdi N, et al. Effects of training in the Morris water maze on the spatial learning acquisition and VAChT expression in male rats. Daru. 2011; 19: 166-172.
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999; 66: 137-147.
- Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adh Migr. 2009; 3: 88-93.
- Balu M, Sangeetha P, Haripriya D, Panneerselvam C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci Lett. 2005; 383: 295-300.
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010; 4: 118-126.
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007; 2: 219-236.
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009; 7: 65-74.
- Younes M, Siegers CP. Mechanistic aspects of enhanced lipid peroxidation following glutathione depletion in vivo. Chem Biol Interact. 1981; 34: 257-266.
- Mulier B, Rahman I, Watchorn T, Donaldson K, MacNee W, Jeffery PK. Hydrogen peroxide-induced epithelial injury: the protective role of intracellular nonprotein thiols (NPSH). Eur Respir J. 1998; 11: 384-391.
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000; 267: 4912-4916.
- Schuessel K, Leutner S, Cairns NJ, Müller WE, Eckert A. Impact of gender on upregulation of antioxidant defence mechanisms in Alzheimer's disease brain. J Neural Transm. 2004; 111: 1167-1182.
- Mann H, McCoy MT, Subramaniam J, Van Remmen H, Cadet JL. Overexpression of superoxide dismutase and catalase in immortalized neural cells: toxic effects of hydrogenperoxide. Brain Res. 1997; 770: 163-168.
- Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain Res Rev. 1995; 21: 285-300.
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999; 49: 921-937.