1Department of Cell and Developmental Biology, University College London, UK
2Spectroscopy Products Division, Renishaw plc, UK
3Biophotonics Research Unit, Gloucestershire Royal Hospital, UK
4Department of Gastrointestinal Pathology, University College Hospital, UK
*Corresponding author: Thomas GM, Department of Cell and Developmental Biology, University College London, Gower Street, London, WC1E 6BT, United Kingdom
Received: November 25, 2014; Accepted: November 27, 2014; Published: November 29, 2014
Citation: Gaifulina R, Lau K, Rodriguez-Justo M, Kendall C and Thomas GM. Light at the End of the Tunnel: Application of Raman Spectroscopy in Colorectal Cancer Diagnostics. Austin J Clin Pathol. 2014;1(5): 1021.
Since its introduction in 1837, by Wissowzky, haematoxylin and eosin (H&E) staining has remained the primary contrasting technique for clinical pathology and tissue-based cancer diagnostics regardless of cancer origin [1]. Over the years it has evolved to become a powerful tool in the diagnostic world and is the most routine and cost-effective approach in use. However, a complete diagnosis is not always made from morphological observation alone hence additional testing may be carried out, this is often by immunohistochemistry (IHC) which was first described in 1942 [2]. This technique exploits the localization and abundance of specific disease markers and in addition to determining tumours of uncertain histogenesis it can assist in predicting the likely response to therapy and prognosis. It is therefore a powerful complementary technique that gives a greater insight into the immunological and biochemical state of the tissue. All these techniques however share one Achilles heel; not only do they all require the application of stains, dyes or tags but pathologists also succumb to inter- and intra-observer variability [3]. Furthermore, target molecules must be known prior to the application of IHC. Importantly there has also been some speculation that the standard chemical processing of biopsies and resections alike causes inconsistencies and artefacts in the staining [4].
Here we outline a technique that can be used as an adjunct to the standard pathological practice. Vibrational spectroscopic techniques – especially Raman Spectroscopy (RS) has demonstrated capability for expanding the boundaries of molecular pathology, particularly in the clinical field [5,6]. Multiple studies have already successfully applied Raman spectroscopy for the discrimination of cancer from healthy tissues, and the colon offers many excellent examples. RS is a technique where additional dimensions of molecular information can be provided alongside the standard visual and targeted biochemical examination currently carried out with H&E and IHC staining. Raman spectral characterization of all the different subgroups of colonic lesions or polyps allows databases of the biochemical features of the tissues to be generated, potentially providing a more refined molecular analysis of the lesion. This will in turn facilitate better predictions of their behaviour. By allowing pathologists to adopt precision RS techniques one can enhance rapid and objective diagnosis, and hence alleviate the current work load pressures exerted on pathologists.
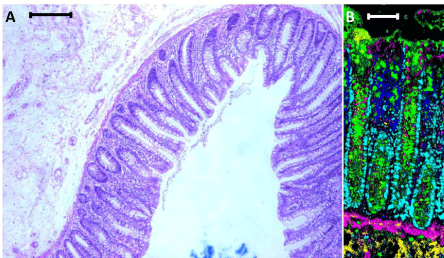
Raman spectroscopy is a vibrational spectroscopic technique that can be adapted for imaging applications enabling the abundance and distribution of the various biochemical constituents within the tissue to be mapped with high spatial resolution. It is therefore not only capable of providing a unique biochemical fingerprint of the tissue but also morphological information. It also provides superior contrast to the traditional H&E staining. Figure 1 shows a H&E stained human colon section, and a false coloured composite image generated using a Renishaw Raman microscope (Renishaw plc, Gloucestershire, UK) utilizing a 785 nm laser line that scans across the tissue section. The different colours correspond to the unique biochemical fingerprint obtained from the different cell types and constituents that comprise the colon tissue section. However, this is only a superficial demonstration of Raman capabilities. Many efforts have already been made in applying Raman spectroscopy in colorectal cancer tissue; most of these studies are focused on cancer discrimination using powerful chemometric approaches [7-14]. One such study has achieved a classification of up to 99.5% sensitivity and specificity in the discrimination of normal and malignant tissues [8]. Furthermore, the approach used is user-friendly and can be applied with no prior Raman or chemometric knowledge. The technique is based on an automated comparison of spectra from the sample against a pre-existing spectral database built from clear-cut diagnostic cases. We would like to direct the reader to a number of excellent reviews in the use of Raman spectroscopy in pathology [15–17].
Raman applications range beyond the simple cancer vs normal analysis of excised tissues but can also be applied for in vivo analysis of cancer margins. Although not yet trialled in colorectal cancer, RS has been successfully applied for in vivo margin delineation during partial mastectomy surgeries [18]. More objective margin assessment first time round will lead to fewer re-excision procedures being required and hence reduced risk of associated complications, reduced overall stress on the patient and reduced total cost per patient. It may also potentially be used to detect cancer onset significantly earlier and prior to the manifestation of gross morphological changes [19]. As a result a more timely and precise diagnosis could be made that would increase patient survival and quality of life.
The standard anatomical pathology laboratory workflow requires that all tissues undergo fixation in 10% neutral buffered formalin and extensive chemical processing prior to paraffin embedding. There has been some speculation that this extensive chemical exposure is associated with poor or variable staining during IHC and even H&E [4]. Formalin fixation is also a notorious contributor towards the fragmentation of DNA and RNA known to impact the efficacy of gene expression analysis [20]. There has been little success in implementing rigorous standardization across all pathology laboratories in order to ameliorate this and it may be an impossible task. Overall one can say that such rigorous chemical treatment casts a shadow over the gold standard techniques established over the years and raises considerable unresolved questions about the reliability of processed tissues for precision advanced diagnostics. Raman on the other hand lends itself as an excellent technique for the detection of structural changes in proteins induced by chemical means. It may therefore be used to track any structural modifications in proteins, DNA/RNA fragmentation and much more during processing. Effects of formalin fixation have also been reported to have negligible effects on vibrational spectral data rendering RS almost unperturbed to chemical processing [21].
In addition to routine tissue analysis RS can potentially provide supplementary diagnostic information through the characterization of colonic mucus. Previously only noted as a histological feature, mucin plays a more central role in cellular growth, differentiation and transformation, and has been implicated in cancer development and pathogenesis [22]. RS has been shown to be able to detect mucus in formalin fixed paraffin embedded tissue and to provide a biochemical characterization. This leads to the possibility of using mucus as an additional diagnostic parameter alongside morphological evaluation of the tissue and metabolic profiling of the cells through RS. Analysis of the mucus retained in the tissue sections following extensive chemical processing may allow for biochemical characterization as our work shows that initial detection is possible (unpublished). Sampling of mucus during colonoscopies may also be an invaluable tool to enhance the accuracy of colorectal cancer diagnosis. Although this has not yet been trialled using Raman spectroscopy we believe that there is a definite scope for this work as characterization of mucin structure has previously been established [23].
It is clear to see that Raman spectroscopy offers a clear way towards a highly objective means of diagnosis through what can be called ‘bulk metabolic profiling’ of tissue and its associated products. The strength of this technique lies in the ability to look beyond the morphology of the tissue and to delve into what is unseen; the biochemical state of the cells. It is therefore imperative that clinicians are aware of this novel platform and are encouraged to lend their extensive experience to developing this platform further. Only then can it be successfully integrated and provide the anatomical pathology community with the necessary support and improved accuracy in diagnostics, which inevitably benefits both the pathologist and the patients.
Panel A: Standard H&E staining of a normal mucosal fold (scale: 250 μm), Panel B: Composite score image of normal colonic crypts generated using Raman spectroscopy (scale: 100 μm). The different colours in the image are used to quickly and easily represent the distinct, aggregated biochemical features of each tissue component without the need to examine specific features of each Raman spectrum (Yellow: submucosa, Magenta: muscularis mucosa, Green: mucus from goblet cells, Blue: lymphocytes, Cyan: cell nuclei). Importantly each pixel in the image is constituted from a distinct Raman spectrum and can therefore be interrogated for precise, specific biochemical information at a later stage if required. Even at a step size of 1μm the spatial resolution is sufficient to allow a pathologist to easily discriminate individual cellular nuclei.