
Editorial
Austin J Clin Pathol. 2015; 2(3): 1035.
Sugar-Coating the Skin
de Jesus TJ and Ramakrishnan P*
Department of Pathology, Case Western Reserve University and University Hospitals Case Medical Center, USA
*Corresponding author: Ramakrishnan P, Department of Pathology, Case Western Reserve University, Wolstein Research Building, 2013 Cornell Road, Cleveland, OH, 44106, USA
Received: September 28, 2015; Accepted: October 03, 2015; Published: November 02, 2015
Abstract
Hyperglycemia is a hallmark symptom of diabetes. Chronic hyperglycemia plays a major role in the pathology of most or all of the secondary complications associated with diabetes, such as cardiovascular diseases, neuropathy, nephropathy and skin disorders. These pathological effects of hyperglycemia are mainly mediated by high glucose-induced, (a) glycation of biomolecules, (b) altered posttranslational glycosylation of proteins and (c) generation of free radicals and reactive oxygen species. These biochemical events lead to skin pathologies such as chronic inflammation, impaired wound healing and risk of melanoma. These skin diseases, on top of being health risks, also mar our appearance, causing high psychological costs. The following mini review highlights the several ways by which hyperglycemia damages the skin crossing the fields of diabetes, glycobiology and dermatology.
Keywords: Skin; Hyperglycemia; Glycosylation; Glycation; Cancer; Diabetes
Introduction
The number of diagnosed cases of diabetes in the United States has been on an uninterrupted rise since 1980 [1]. According to the Centers for Disease Control and Prevention (CDC) there are over 29 million diagnosed cases of diabetes in the United States [2]. Diabetic patients also develop several secondary complications such as retinopathy, neuropathy, nephropathy, cardiovascular diseases as well as end organ dysfunction [1]. These secondary complications of diabetes are by and large linked to the deleterious effects of chronic hyperglycemia. Besides these complications, hyperglycemia also affects skin, the largest organ of the human body. It plays a role in a wide range of topical and subcutaneous skin disorders, which is a major health concern for diabetics. In 2010, the number of adults with diabetes suffering from concurrent skin disorder was about 180,000, while the number of adults diagnosed with metabolic, nutritional, and immune disorders combined was only 187,000 [1]. There exists a major gap of knowledge on the underlying molecular mechanisms that regulate hyperglycemia-induced secondary complications in the skin and the role of immune system in this process. Here we attempt to link together some existing pieces of information on the potential molecular mechanisms associated with hyperglycemia-induced skin pathologies.
Glycation
Glycation is the non-enzymatic covalent bonding of a reducing sugar, such as glucose to protein, nucleic acid, or lipid molecules that leads to the formation of compounds called Advanced Glycation End-products (AGE) [3]. It forms naturally inside the body through chemical reactions over the course of several hours. Its prevalence depends on several factors such as duration of conditions such as hyperglycemia and protein half-life [4]. The complex sugar-protein or sugar-lipid molecules can bind to Receptor for AGEs (RAGE) on many cell-types, including those cell-types affected by diabetes [5]. AGE signaling and its role in diabetes is one of the better-studied aspects of hyperglycemic complications and its effect on cellular functions. Protein glycation and AGE formation have been found to occur concurrently with the formation of free radicals, which can result in protein fragmentation and DNA damage [6]. Furthermore, skin collagen glycation is not only associated with aging, but has been shown to be accelerated in diabetes, which results in enhanced lowdensity lipoprotein uptake [7].
Wound healing involves keratinocytes, platelets, macrophages, endothelial cells, and fibroblasts. In healthy individuals, these cells release a variety of cytokines and chemokines that facilitate wound healing [8]. For example, epithelial cells and macrophages release factors such as Vascular Endothelial Growth Factor (VEGF) that induce endothelial Nitric Oxide Synthase (eNOS) activation. It leads to the recruitment of Endothelial Progenitor Cells (EPCs) by way of SDF-1α homing to the site of injury for the purpose of vasculogenesis [9]. In diabetic patients, both eNOS production and SDF-1α expression are impaired, which together prevents endothelial replacement at the site of injury [10]. In addition, it has been shown that Fibroblast Growth Factor-2 (FGF-2) is glycated in vivo in mice. The glycation impairs angiogenic properties of FGF-2 further delaying wound healing [11].
The influx of inflammatory cells to the site of injury is initially delayed in diabetic patients. However, once these cells arrive, they maintain their position around the site of injury, resulting in a chronic inflammatory state. Chronic inflammation results in the excessive release of pro-inflammatory molecules and Matrix Metalloproteinase’s (MMPs) that delay wound closure [12]. The sustained inflammatory response has been shown to be in part a result of AGE/RAGE interaction. A greater understanding of the mechanisms behind AGE formation and RAGE signaling cascades will be imperative to design future therapeutic strategies to treat secondary skin disorders associated with diabetes. Impaired wound healing is a significant cause of morbidity and is particularly life-threatening post-surgery among diabetic patients [13]. Thus, understanding the role of glycation in wound healing and angiogenesis is an important area that deserves further investigation.
AbsProtein Glycosylationtract
The long-term increase in environmental sugar levels leads to a global increase in glycosylation events. Glycosylation is a posttranslational modification characterized by the enzymatically catalyzed covalent bonding of carbohydrates to specific amino acids. Protein glycosylation includes the attachment of O-linked, N-linked and C-type glycans, as well as glycosyl phosphatidylinositol attachment (glypiation), catalyzed by various glycosyl transferases [14]. About half of the proteins expressed in a given cell at any time are glycosylated to varying degrees in the lumen of the endoplasmic reticulum or the golgi apparatus of the cell. The exception to this rule being O-GlcNAcylation, which is the addition of a monomer β-N-Acetyl-D-Glucosamine (GlcNAc) to a serine, threonine or tyrosine residue. This type of glycosylation is reversible and occurs in the cytoplasm and nucleus. The addition of GlcNAc to proteins is catalyzed by the enzyme O-GlcNAc Transferase (OGT) and O-GlcNAcase (OGA) mediates its removal from proteins [15,16]. There is opportunity for interference and/or synergy between O-GlcNAcylation and phosphorylation because they target the same amino acids for modification. One study showed direct involvement of O-GlcNAc Transferase with wound healing. Inhibition of OGT was shown to promote wound healing in diabetic mice and a correlation was observed between hyperglycemia, increased O-GlcNAcylation, and a hyper-adhesive keratinocytes phenotype [17].
N-linked glycosylation is the attachment of a glycan to an amide group, and O-linked glycosylation is the attachment of a glycan to a hydroxyl group. Both modifications increase under hyperglycemic conditions and have been shown to be involved in a variety of cellular processes, including cell-cell interaction, cell survival/death and growth/proliferation. In 2008, it was demonstrated that up regulation of N-linked glycosylation of pro-inflammatory and cell survival proteins was associated with skin cancers in mice [18]. Additionally, sites of O-linked glycosylation on proto/ontogenesis have been shown to be sites of frequent mutation in both skin cancers and lymphomas [19]. Study of these specific, enzymatically driven glycosylation events could open up new avenues for treatment of hyperglycemiaassociated skin complications.
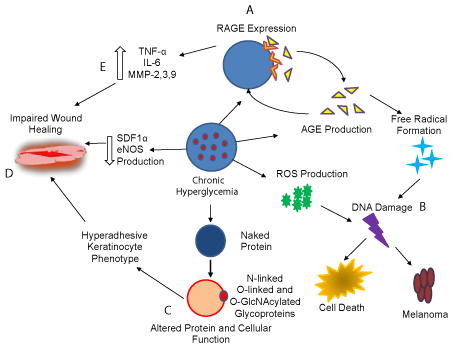
Figure 1: A summary of selected hyperglycemia associated complication
that affects skin (described clock wise). A. Hyperglycemia triggers the
production of Advanced Glycation End Products ( AGE), which binds to their
receptor (RAGE) triggering free radical formation, damaging DNA which
either kills the cell or cause tumorigenesis. B. Hyperglycemia also triggers
ROS production from mitochondria which cause DNA damage leading to
cell death or oncogenic transformation. C. Hyperglycemia increases the
pool of glycoproteins such as O-GlcNAcylated proteins that may alter protein
function, cell survival, death and differentiation. D. Hyperglycemia reduces
the production of eNOS and SDF 1α, which contributes to impairment of
wound healing. E. Hypoglycemia enhances the production of proinflammatory
mediators causing chronic inflammation and impaired wound healing.
Hyperglycemia and Skin Cancer
Several studies reported over the last decade implicate an association between hyperglycemia and tumorigenesis [20, 21]. Indeed, diabetes-induced hyperglycemia has been shown to play a role in many different cancer subtypes. A 2004 epidemiological study showed that diabetes patients with 15+ years of disease showed a nearly 2-fold increase in the prevalence of all cancer subtypes [20]. Additionally, there was a correlation shown between current insulin use and increased incidence of non-melanoma skin cancers [22]. Furthermore, chronic high blood-glucose has been correlated with a 41% increase in mortality in patients with breast, colorectal, and uterine cancer [23]. Increased glucose levels also result in an increase in mitochondrial Reactive Oxygen Species (ROS) and free radical production. Since these chemically reactive molecules damages cellular DNA, it has been suggested that hyperglycemia can lead to increased tumorigenesis [24]. Understanding the alternation and the causation of these events could help develop treatments for glycosylation associated skin cancers.
Conclusion
Hyperglycemia has broad, deleterious effects on all organ systems of the body. The skin, is not only the largest of the organs, but keeps the rest of the body separate from the external environment. The skin suffers both external and internal assaults. Therefore its maintenance and immune regulation is key to a healthy life. Glycosylation and glycation events act as a direct link between nutritional intake and cellular function, with their levels changing with the presence of glucose in the bloodstream. Keratinocytes and skin fibroblasts have many proteins that are glycosylation targets both within the cell and secreted into the extra cellular matrix. These modifications effect protein functions that control secretion, survival, and migration of cells, all of which are important to the maintenance of skin as an exterior organ. People suffering from chronic hyperglycemia suffer from debilitating limitations and high medication costs. Concurrent skin diseases also come with a high psychological cost, often leading patients to become social pariahs. The incidence of type 1 and type 2 diabetes as well as obesity is on the rise and all of these diseases result in hyperglycemia. Hence, there is an immediate need to understand the molecular mechanisms mediating hyperglycemia-associated complications of the skin, the defensive shield that protects our health and preserves our beauty.
References
- Center for Disease Control and Prevention. Basics about Diabetes.
- Center for Disease Control and Prevention. National Diabetes Statistics Report. 2014.
- Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981; 213: 222-224.
- Giardino ID, Edelstein, Brownlee M. Non enzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994; 94: 110-117.
- Xie J. Cellular signalling of the Receptor for Advanced Glycation End Products (RAGE). Cell Signal. 2013; 25: 2185-2197.
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991; 40: 405-412.
- Brownlee MH, Vlassara, Cerami A. Non enzymatic glycosylation products on collagen covalently trap low-density lipoprotein. Diabetes. 1985; 34: 938-941.
- Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemo taxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000; 102: 185-190.
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007; 117: 1219-1222.
- Abaci A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999; 99: 2239-2242.
- Facchiano F. Glycated fibroblast growth factor-2 is quickly produced in vitro upon low-mill molar glucose treatment and detected in vivo in diabetic mice. Mol Endocrinal. 2006; 20: 2806-2818.
- Giacomoni PU, Rein G. Factors of skin ageing share common mechanisms. Bio gerontology. 2001; 2: 219-229.
- Spravchikov N. Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes. 2001; 50: 1627-1635.
- Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012; 13: 448-462.
- Hart GW. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription and chronic disease. Annu Rev Biochem. 2011; 80: 825-858.
- Ramakrishnan P. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci Signal. 2013; 6: 75.
- Runager K. Targeting O-Glycosyltransferase (OGT) to promote healing of diabetic skin wounds. J Biol Chem. 2014; 289: 5462-5476.
- Tian Y, Zhang H. Characterization of disease-associated N-linked glycoproteins. Proteomics. 2013; 13: 504-511.
- Chen L. Interference with O-glycosylation in RMA lymphoma cells leads to a reduced in vivo growth of the tumor. Int J Cancer. 2006; 119: 1495-1500.
- Coughlin SS. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004; 159: 1160-1167.
- Barone BB. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008; 300: 2754-2764.
- Li C. Prevalence of diagnosed cancer according to duration of diagnosed diabetes and current insulin use among U.S. adults with diagnosed diabetes: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care. 2013; 36: 1569-1576.
- Duan W. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014: 4619-4617.
- Li W. Hyperglycemia enhances the invasive and migratory activity of pancreatic cancer cells via hydrogen peroxide. Oncol Rep. 2011; 25: 1279-1287.