
Research Article
J Dent & Oral Disord. 2018; 4(1): 1081.
Roles of EphrinB2 and EphB4 in Alveolar Bone under Initial Compressive Mechanical Stress of Dental Implant Replacement
AKitamura K¹*, Mine Y², Koto W¹, Wachi T¹, Shinohara Y¹, Makihira S¹ and Koyano K¹
¹Department of Oral Rehabilitation, Faculty of Dental Science, Kyushu University, Maidashi, Japan
²Department of Research and Development of Next Generation Medicine, Faculty of Medical Sciences, Kyushu University, Japan
*Corresponding author: Kazuyuki Kitamura, Section of Fixed Prosthodontics, Department of Oral Rehabilitation, Faculty of Dental Science, Kyushu University, Maidashi, Higashi-ku, Fukuoka, Japan
Received: December 01, 2017; Accepted: January 08, 2018; Published: January 15, 2018
Abstract
The communication between ephrin/Eph families was recently shown to be involved in cell differentiation under mechanical stress. To understand the roles of EphrinB2 and EphB4 in osteoblast cells and alveolar bone under initial mechanical stress of dental implant replacement, first, MC3T3-E1 cells were subjected to cyclical compressive or extensive force in vitro. The expressions of EphrinB2 and EphB4 were examined by real-time RT-PCR or western blotting, together with expression of Osterix as an osteoblast differentiation marker. Next, the effects of mini-implant surfaces immobilised with EphrinB2 or EphB4 on the expression of the gene encoding Osterix in alveolar bone surrounding implants were examined by real-time RT-PCR. Osterix mRNA expression was decreased in MC3T3-E1 cells cultured under compressive conditions, compared with that under normal conditions. The mRNA and protein levels of EphrinB2 and EphB4 were increased in the cells cultured under compressive conditions. Initially, mechanical stress by torques decreased Osterix mRNA expression in alveolar bone surrounding mini-implants, compared with the control groups. Torque of 10 Nmm increased EphB4 mRNA expression compared with the control groups, while torques of 10 and 20 Nmm increased EphrinB2 mRNA expression. The mini-implant surface immobilised with EphrinB2-Fc recovered the suppression of Osterix. Taken together, the present results suggest that increased binding of EphrinB2 and EphB4 in osteoblast cells exposed to initial mechanical force, especially compressive force, may be involved in alveolar bone recovery, and may cause a gradual decrease of the primary fixation in a replaced dental implant and finally lead to stability.
Keywords: Compressive stress; EphrinB2-EphB4; Extensive stress; Implant
Abbreviations
Mini-Implant: Titanium Mini-Implant; Ti-Disc: Titanium Disc; XPS: X-Ray Photoelectron Spectroscopy; CL: Cell Lysate
Introduction
Recent investigations have demonstrated that molecules in the ephrin/Eph family regulate the differentiation of osteoblasts and osteoclasts under mechanical stress [1]. Ephrins and Ephs have a ligand–receptor relationship. Ephrins are transmembrane ligands, and Ephs are their tyrosine kinase receptors. Interactions between ephrinB- and EphB-expressing cells result in bidirectional signal transduction [2]. Activation of EphB receptors by ephrinB ligands is referred to as “forward signaling”, while activation of ephrinB ligands by EphB receptors is designated “reverse signaling” [2].. The communication between EphrinB2 and EphB4 was recently shown to be involved in the stimulation of osteoblast differentiation within the osteoblast lineage 2. In addition, EphrinB2 and EphB4 were reported to be regulated by mechanical forces in endothelial progenitor cells and periodontal ligament fibroblasts [1]. Meanwhile, ephrinA2-EphA2 interactions may be involved in the initiation phase of bone remodelling by enhancing osteoclast differentiation and suppressing osteoblast differentiation [3]. However, there have been no investigations on ephrin and Ephmolecules, including ephrinA2, EphrinB2, EphA2, and EphB4, in the surrounding bone under compressive mechanical stress environments after replacement of a dental implant.
Deformation and elastic deformation of the implant body after implantation, early osseointegration acquisition to the implant surface, and biological responses of surrounding structures to mechanical stresses related to a decrease in the primary fixation are involved in successful achievement of safe stability of an implant in alveolar bone [4]. In this study, we investigated the roles of EphrinB2 and EphB4, as known molecules related to osteoblast and osteoclast differentiation under mechanical forces, in remodelling of the bone tissue surrounding an implant under compressive and extensive mechanical stress environments arising from the implantation, to understand the biological behaviors’ of alveolar bone in the initial mechanical stress using a rat dental implant model.
Material and Methods
Materials
Recombinant rat EphrinB2-Fc and EphB4-Fc were obtained from R&D Systems (Minneapolis, MN). Pure wrought Titanium discs (Tidiscs; JIS, Japan Industrial Specification H 4600, 99.9 mass% Ti, 15- mm diameter; Kobelco, Kobe, Japan) were used to confirm the Tisurface modification of mini-implants by EphrinB2-Fc and EphB4- Fc. The Ti-discs were sandblasted (HI ALUMINAS; Shofu, Kyoto, Japan) before surface modification. Pure Titanium mini-implants (mini-implants; 2-mm diameter, 4-mm length; Kondo Technology, Tokyo, Japan) were used for the in vivo study (Appendix Figure 1). The Ti-discs and mini-implants were washed with acetone and ethanol in an ultrasonic bath for 30 min and autoclaved before use.
Cell culture under normal, compressive, and extensive conditions
The MC3T3-E1 cell line was purchased from the European Collection of Cell Cultures (ECACC, Wiltshire, UK). MC3T3-E1 cells were cultured in a-MEM supplemented with an antibiotic mixture (Thermo Fisher Scientific, Waltham, MA), 10% fetalbovine serum (Biological Industries, Haemek, Israel), and 50 μg/mL L-ascorbic acid (Merck, Darmstadt, Germany) at 37°C under 5% CO2/95% humidified air. During culture, the medium was refreshed at 3-day intervals, unless otherwise required for specific experiments. One group of MC3T3-E1 cells (normal conditions; Group-N) was maintained in the conventional environment described above, while two other groups (compressive conditions: Group-C; extensive conditions: Group-E) were cultured under cyclical compressive stress and extensive stress, respectively. A loading device (STB-140; STREX, Tokyo, Japan) was used to create the two types of stress.
Immobilisation of EphrinB2-Fc or EphB4-Fc on the surfaces of Ti-discs and mini-implants
Immobilisation of EphrinB2-Fc or EphB4-Fc on Ti surfaces was carried out as described previously [5]. Briefly, Ti-discs were immersed in 5% γ-aminopropyltriethoxysilane in acetone for 15 min at room temperature, washed with acetone, treated with 5% glyoxylic acid monohydrate for 2 h, and washed with ultrapure water. The surfaces of the specimens were then treated with 0.4% sodium borohydride for 24 h to reduce the imine groups to amine groups. After this series of pre-treatments, the specimens were washed with ultrapure water and autoclaved. The carboxyl groups on the specimen surfaces were activated with N-hydroxylsuccinimide/N-ethyl-N’- (3-dimethylaminopropyl)-carbodiimide (Biacore AB, Uppsala, Sweden), and then treated with 20 μg/ml EphrinB2-Fc or EphB4-Fc in sodium bicarbonate buffer (pH 8.0) for 30 min at 37°C and 16 h at 4°C to immobilise EphrinB2-Fc or EphB4-Fc on the surface, creating EphrinB2-Fc-Ti and EphB4-Fc-Ti, respectively. After washing with phosphate-buffered saline to remove any excess EphrinB2 or EphB4, the activated carboxyl groups were blocked by treatment with 1 M ethanolamine-HCl (Biacore AB) for 5 min. Untreated Ti-discs were used as control specimens (control-Ti). The body surfaces of the mini-implants were immobilised with EphrinB2-Fc or EphB4-Fc using the same method described above for the Ti-discs.
Implantation of mini-implants into the rat palatine process
Eight-week-old Wistar rats (Kyudo Co. Ltd., Fukuoka, Japan) were used for the animal study. The animal selection, management, anesthesia, surgery, and analysis procedures were approved by the Animal Care and Use Committee of Kyushu University (Approval No. A25-138). All in vivo experiments using the rat model were performed in accordance with the procedures allowed by the committee. Briefly, the rats were carefully anaesthetized with sevoflurane and pentobarbital. Prior to surgery, the implantation site was disinfected with 10% iodine. During the surgery, a mini-implant was placed into the palatine process of the maxilla using micro drivers, and the recipient gingival site was tightly sutured. The initial torques for implantation was 10, 20, and 30 Nmm. Each animal received only one implant. For real-time RT-PCR, the alveolar bone from the samples was collected after 3 h. Two independent experiments involving triplicate samples were performed.
Real-time RT-PCR analysis
Total RNA was extracted from homogenates using TRIzol (Thermo Fisher Scientific). First-strand cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan) with 100 ng of total RNA. The cDNA was amplified by BIOTAQ DNA polymerase (Bioline, Randolph, MA). Real-time RT-PCR analysis for Osterix, ephrinA2, EphrinB2, EphA2, EphB4, and β-actin was performed using a Rotor-Gene™ 6000 (Qiagen, Tokyo, Japan). β-actin was chosen as an internal control to standardize the variability in amplification arising from slight differences in the starting total RNA concentrations. The sequences of the primers and probes are listed in Appendix Tables 1 and 2. The sequences of the primers and probesfor β-actin were described previously [6].
Western blotting
Cell lysates (CLs) from cultured MC3T3-E1 cells were lysed in Laemmli buffer and stored overnight at -80°C. After centrifugation of the CLs at 15,000×g for 20 min at 4°C, the supernatants were transferred to new microtubes, boiled for 5 min, and stored at -20°C. The proteins present in the CLs were separated in SDS-PAGE gels and transferred to PVDF membranes (Immuno-Blot PVDF Membranes; Bio-Rad, Hercules, CA). The membranes were probed with primary antibodies against EphrinB2 (R&D Systems) and EphB4 (Proteintech, Wuhan, China), followed by incubation with antigoat IgG conjugated with horseradish peroxidase and anti-rabbit IgG conjugated with horseradish peroxidase (Abcam, Cambridge, UK) for detection of EphrinB2 and EphB4, respectively. Immune complexes containing horseradish peroxidase bound to the target molecules on the PVDF membranes were detected by an enhanced chemiluminescence detection kit (ECL plus Western Blotting Detection System; GE Healthcare, Buckinghamshire, UK).
X-ray photoelectron spectroscopy
To evaluate immobilized fusion proteins (EphrinB2-Fcand EphB4-Fc), the surface chemical compositions of treated samples were analysed by X-ray photoelectron spectroscopy (XPS; K-alpha; Thermo Fisher Scientific). Monochromatised Al Ka X-rays at 1486.6 eV were used for excitation under 2.0×10-7 Pa. The spot size of the incident X-rays was 400μm in diameter. The XPS machine was calibrated using Au 4f7/2 at 83.96 eV and Ag 3d5/2 at 368.21 eV. For survey scans, the detector was set at 200 eV as passing energy and 10 ms as dwell time. For narrow scans, the detector was set at 50 eV and 50 ms, respectively. All measurements were referenced by setting the hydrocarbon of C 1s to 284.8 eV. Detected elements were quantified using Avantage version 5.36 software (Thermo Fisher Scientific).
Data analysis
The significance of differences in the values of two groups was assessed by Student’s t-test. Differences among the mean values of the groups were subjected to one-way analysis of variance (ANOVA) and Tukey’s multiple-range test. SPSS version 20.0 software (IBM, Tokyo, Japan) was used for the statistical analyses.
Results
mRNA expression levels of Osterix, ephrins, and Ephs in MC3T3-E1 cells stimulated by cyclic stress
The effects of cyclic compressive or extensive stress (5% and 10%) on osteoblast differentiation were monitored using the MC3T3-E1 osteoblastic cell line for up to 18 h. First, the expression levels of an osteoblast-specific gene transcription marker, Osterix, were analyzed to evaluate and standardize the differentiation stage of MC3T3-E1 cells under normal, compressive, and extensive conditions during culture periods of 1 to 18 h after the cells had reached confluence. β-actin was chosen as an internal standard to control for variability in amplification arising from differences in the starting total RNA concentrations.
The real-time RT-PCR results showed that the expression levels of Osterix mRNA were decreased in cells cultured under 5% compressive stress (Group-5C) for 1, 3, and 18 h, compared with cells cultured under normal conditions (Group-N) (Student’s t-test, p<0.05; Figure 1A). Similar results for the expression levels of Osterix mRNA were obtained for cells cultured under 5% extensive stress (Group-5E) compared with Group-N cells (Student’s t-test, p<0.05, p<0.01; Figure 1B).
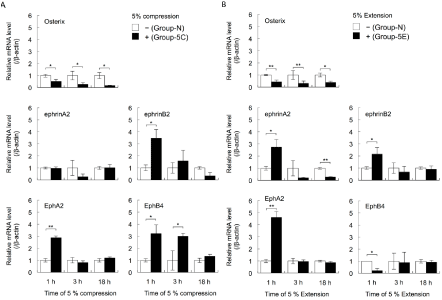
Figure 1: The effects of cyclical compressive stress (A: 5% compression) and extensive stress (B: 5% extension) on the expression levels of the genes encoding
Osterix, ephrinA2, EphrinB2, EphA2, and EphB4 in MC3T3-E1 cells were analysed by real-time RT-PCR. At confluence, the cells were exposed to 5% cyclical
compressive stress or extensive stress for 1, 3, or 18 h. Total RNA was isolated from the cells in each condition and subjected to real-time RT-PCR analysis. The
RT-PCR data for the expression levels of Osterix, ephrinA2, EphrinB2, EphA2, and EphB4 mRNAs were normalised by the expression levels of β-actin mRNA.
Two independent experiments involving triplicate samples were performed. Representative data from the two experiments are shown as means ± SD. *p<0.05,
**p<0.01.
Next, the expression levels of ephrinA2, EphrinB2, EphA2, and EphB4 mRNAs in MC3T3-E1 cells were investigated. Culture under 5% compressive stress caused no changes in ephrinA2 expression in cells after 1, 3, and 18 h (Group-5C vs. Group-N; Figure 1A). However, the expression levels of EphA2 mRNA in Group-5C cells were increased at 1 h compared with the levels in Group-N cells (Student’s t-test, p<0.01; Figure 1A). Compared with Group-N cells, the expression levels of EphrinB2 mRNA at 1 h and EphB4 mRNA at 1 and 3 h were increased in Group-5C cells (Student’s t-test, p<0.05; Figure 1A). The expression levels of ephrinA2, EphrinB2, and EphA2 mRNAs in cells exposed to 5% extensive stress (Group-5E) were increased at 1 h, compared with the levels in Group-N cells (Student’s t-test, p<0.05, p<0.01; Figure 1B). However, extensive stress for 18 h significantly decreased the expression levels of ephrinA2 mRNA. The expression levels of EphB4 in Group-5E at 1 h were significantly decreased (Student’s t-test, p<0.05, p<0.01; Figure 1B).
Exposure to 10% compressive or extensive stress decreased the expression levels of Osterix and EphrinB2 mRNAs in MC3T3-E1 cells (Student’s t-test, p<0.05, p<0.01; Figure 2). Culture under 10% compressive stress for 1 h significantly increased the expression levels of EphA2 and EphB4 mRNAs in MC3T3-E1 cells (Student’s t-test, p<0.05, p<0.01). In contrast, culture under 10% extensive stress for 1 h significantly decreased the expression levels of EphA2 and EphB4 mRNAs in MC3T3-E1 cells (Student’s t-test, p<0.01). Furthermore, culture under 10% extensive stress for 1 h increased the expression levels of ephrinA2 mRNA (Student’s t-test, p<0.01; Figure 2).
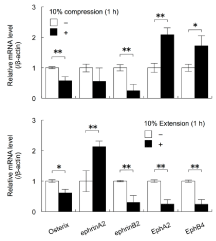
Figure 2: The effects of cyclical compressive stress (A: 10% compression)
and extensive stress (B: 10% extension) on the expression levels of the genes
encoding Osterix, ephrinA2, EphrinB2, EphA2, and EphB4 in MC3T3-E1 cells
were analysed by real-time RT-PCR. At confluence, the cells were exposed
to 10% cyclical compressive stress or extensive stress for 1, 3, or 18 h.
Total RNA was isolated from the cells in each condition and subjected to
real-time RT-PCR analysis. The RT-PCR data for the expression levels of
Osterix, ephrinA2, EphrinB2, EphA2, and EphB4 mRNAs were normalised
by the expression levels of β-actin mRNA. Two independent experiments
involving triplicate samples were performed. Representative data from the
two experiments are shown as means ± SD. *p<0.05, **p<0.01.
Protein levels of EphrinB2 and EphB4 in MC3T3-E1 cells cultured under compressive stress
The western blotting results showed that the protein levels of EphrinB2 and EphB4 in CLs from Group-5C were increased compared with the levels in CLs from Group-N (Figure 3). However, there was no difference in β-actin expression in CLs from cells cultured with and without 5% compressive stress (Figure 3).

Figure 3: The effects of cyclical compressive stress and extensive stress (5%
compression) on the protein levels of EphrinB2 and EphB4 in Cell Lysates
(CLs) were examined by western blotting. When MC3T3-E1 cells reached
confluence, the cells were exposed to 5% cyclical compressive stress for 24
h. The proteins in the CLs from control and stressed cells were isolated with
Laemmli buffer. Western blotting was performed using specific antibodies
against EphrinB2, EphB4, and β-actin. Independent experiments were
repeated twice and representative data from the two experiments are shown.
Effects of EphrinB2-Fc or EphB4-Fc on expression levels of Osterix mRNA in MC3T3-E1 cells with or without a medium change after exposure to 5% compressive stress
The expression levels of Osterix mRNA were increased in cells continuously cultured for 3 h under the normal conditions after exposure to cyclical 5% compressive stress for 18 h, compared with the levels in cells exposed to cyclical 5% compressive stress for 3 or 18 h (ANOVA, p<0.05; Figure 4). The expression levels of Osterix mRNA in cells cultured under the normal conditions for 3 h with a medium change after exposure to cyclical 5% compressive stress for 18 h were similar to the levels in cells exposed to cyclical 5% compressive stress for 21 h (Figure 4). Addition of EphrinB2-Fc enhanced the expression levels of Osterix mRNA that had been downregulated by cyclical 5% compressive stress, regardless of whether there was a medium change (ANOVA, p<0.01; Figure 4).
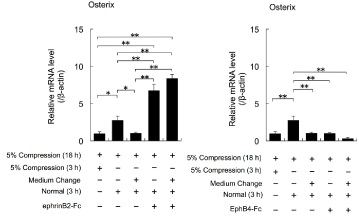
Figure 4: The effects of cyclical compressive stress (5% compression) on
the expression levels of Osterix mRNA in MC3T3-E1 cells were analysed
by real-time RT-PCR. At confluence, the cells were exposed to 5% cyclical
compressive stress for 18 h. The cells were then cultured in the presence
or absence of EphrinB2-Fc or EphB4-Fc for 3 h with or without a medium
change before exposure to EphrinB2-Fc or EphB4-Fc. Representative data
from two experiments are shown as means ± SD. *p<0.05, **p<0.01 vs. 5%
compression (3 h or 18 h).
Meanwhile, addition of EphB4-Fc had no effects on the expression levels of Osterix mRNA that had been downregulated by cyclical 5% compressive stress, regardless whether there was a medium change (ANOVA, p>0.01; Figure 4).
Effects of mini-implant implantation on the expressions of Osterix, EphrinB2, and EphB4 in ratalveolar bone
Torques of 10, 20, and 30 Nmm maintained for 3 h significantly decreased the expression levels of Osterix mRNA in alveolar bone surrounding the mini-implants (Figure 5A), compared with the levels in control groups that contained implant cavities but no miniimplants (ANOVA, p<0.05; Figure 5B). Torques of 10 and 20 Nmm maintained for 3 h increased the expression levels of EphrinB2 mRNA, compared with the levels in the control groups (ANOVA, p<0.01; Figure 5B). A torque of 10 Nmm maintained for 3 h increased the expression levels of EphB4 mRNA, compared with the levels in the control groups (ANOVA, p<0.05; Figure 5B).
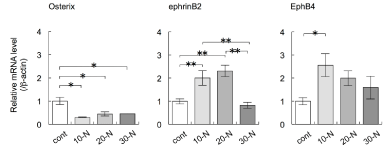
Figure 5: The effects of various torques during mini-implant implantation into the palatine process of the rat maxilla on the expression levels of the genes encoding
Osterix, EphrinB2, and EphB4 in alveolar bone surrounding the mini-implants were examined by real-time RT-PCR. The mini-implants were placed into the palatine
process with torques of 10, 20, and 30 Nmm and maintained for 3 h. Total RNA was isolated from each sample and subjected to real-time RT-PCR analysis. The
expression levels of the target mRNAs were normalised by the expression levels of β-actin mRNA. Two independent experiments involving triplicate samples were
performed. Representative data from the two experiments are shown as means ± SD. *p<0.05, **p<0.01.
Next, Ti-discs were immobilized with EphrinB2-Fc or EphB4-Fc. The elements on the disc surfaces, including Ti, C, O, and N, were analysed by XPS. The XPS results revealed that N was detected on the surfaces immobilized with EphrinB2-Ti and EphB4-Ti. Meanwhile, N was not detected on the surface of control-Ti (Appendix Table 3). The mini-implants in Figure 5A were immobilized with EphrinB2- Fc or EphB4-Fc using the same method applied to the Ti-discs. Implantation of mini-implants immobilized with EphrinB2-Fc on their surfaces changed the expression levels of Osterix mRNA to similar levels to those observed with drilling alone (Figure 6). Meanwhile, the expression levels of Osterix mRNA did not differ significantly in the groups with or without immobilized EphB4-Fc (ANOVA, p>0.05; Figure 6).
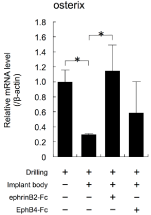
Figure 6: The effects of implantation of mini-implants with immobilised
EphrinB2 or EphB4 on their surfaces on the expression levels of the gene
encoding Osterix in alveolar bone surrounding the implants were examined
by real-time RT-PCR. Mini-implants were placed into the palatine process of
the rat maxilla with a torque of 10 Nmm and maintained for 3 h. Total RNA was
isolated from each sample and subjected to real-time RT-PCR analysis. The
expression levels of the target mRNAs were normalised by the expression
levels of β-actin mRNA. Two independent experiments involving triplicate
samples were performed. Representative data from the two experiments are
shown as means ± SD. *p<0.05, **p<0.01.
Discussion
There are various methods for loading mechanical stress onto cultured cells to simulate mechanical stress conditions in vivo, including the four-point bending system [7], placement of a silicone cylinder on a cell layer cultured on a plate [8], and a stretch chamber system attached to a stretching apparatus that can be driven by a computer-controlled motor [9]. Among these, the system using the stretch chamber with loading in one axis direction 9 was chosen for this study, because reproducibility was considered very important.
We focused on investigating the functions of EphrinB2 and EphB4 in alveolar bone tissue using the stretch chamber system and a rat model involving mini-implants to understand the biological behaviors of osteoblast cells and alveolar bone under mechanical stress. First, an in vitro study was performed to investigate the effects of compressive and extensive forces on Osterix, ephrinA2, EphrinB2, EphA2, and EphB4 in cultured osteoblastic MC3T3-E1 cells [10] using the stretch chamber system [9]. Both compressive force and extensive force suppressed initial osteoblast differentiation. Furthermore, ephrinA2, EphrinB2, EphA2, and EphB4 all showed changes in their expression levels. In particular, EphB4 expression was reversed by both compressive force and extension force. Even when the magnitude of stimulation was increased, the same results were obtained.
To investigate the loaded state immediately after implant implantation, an animal model involving implantation of a miniimplant into the rat palatine process [11] was selected for this study. As the effects of tooth extraction were avoided, it was possible to exclude factors other than inflammation caused by drilling, and to compare the states immediately after implantation [12]. Using this model, several experiments were carried out.
The conditioned medium from cultured cells stimulated by compressive force contained various factors including soluble ephrin and Eph family members. The down regulated differentiation by compressive force was recovered by incubation under normal conditions without a medium change. The interactions between secreted EphrinB2 from and membrane-type EphB4 on the osteoblastic MC3T3-E1 cells induced by compressive stimulation may have been involved in this recovery (Figure 7), because addition of EphrinB2-Fc similarly recovered the osteoblast differentiation in the presence of both compressive stimulation and a medium change. Autocrine and paracrine communication of EphrinB2 and EphB4 expressed by osteoblasts under compressive force may be important for the regulation of osteoblast differentiation. EphrinB2 exists as a membrane type and a secreted type, and its signals are transmitted in both directions upon binding between membrane- type EphrinB2 molecules [13]. It is suggested that EphrinB2 secreted into the medium, but not membrane-type EphrinB2, may be active around osteoblast cells. Signal transduction through EphrinB2, which activates osteoclast differentiation, was not important. This may be related to the fact that macrophage activation is more prominent than osteoclast activation immediately after implant implantation [14].
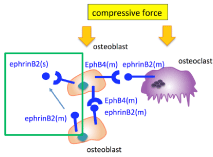
Figure 7: Schematic illustration. Secreted EphrinB2, EphrinB2 (s), from the
cell surface of osteoblast cells binds to membrane-type EphB4, EphB4 (m),
on the cell membrane of the same cells under compressive force.
According to the results of the in vivo study, the expression levels of EphrinB2 and EphB4 were increased by torque loading. In addition, implantation of EphrinB2-Fc-coated mini-implants predominantly restored osteoblast differentiation, which was otherwise decreased by mini-implant implantation. This suggests that EphB4, a receptor for EphrinB2, may be involved in the response of the surrounding bone tissue to the initial mechanical stress after implant implantation. These interactions of soluble EphrinB2 and membrane-type EphB4 may lead to a gradual decrease of the first fixation under torque loading (Figure 7).
The total fixation of an implant is the sum of the primary mechanical fixation force (first fixation) and the fixation force obtained by osseointegration acquisition (second fixation) [4,15,16]. The ratio of the first fixation and the second fixation changes, until stability contributed by the second fixation alone finally supports the implant [4,17]. In the first fixation, the initial mechanical stress from the implantation torque loading acts on the tissues surrounding the implant as well as on the implant itself. The structures surrounding the implant are considered to affect the subsequent changes in the primary fixation force [18]. Meanwhile, in the second fixation, numerous research and development studies on modifications of implant surfaces have been carried out, because sophisticated surfaces may achieve earlier osseointegration acquisition [19,20]. Therefore, balanced behaviors of the implant itself and the surrounding structures, involving a gradual decrease in the first fixation and early acquisition of osseointegration, result in safe stability without loss of implants. Infection control is another important factor for long stability.
Deformation and elastic deformation provoked in both the implant body and the surrounding structures during and after implantation may be dependent on the strength of the torque. However, no fractures of implant bodies were reported for application of the maximum torques required during replacement of modern dental implants [21]. On the contrary, insufficient torques may lead to failure of osseointegration and loss of the first fixation [22].
At the interface between the surface of the implant and the surrounding tissues including bone, epithelium, and connective tissues, biological responses to the initial mechanical stress occur and osseointegration starts. The primary responses cause a gradual decrease in the first fixation and lead to the second fixation by osseointegration acquisition [23]. Therefore, it is important to investigate the behaviors’ at the micro-level not only in the implant body, but also in the surrounding tissues under mechanical stress caused by torques, as well as those at the macro-level to predict and assess the temporal alterations in the sum of the first fixation and the second fixation (osseointegration).
When industrial screws and dental implant bodies were compared, the primary fixation of the bone around the implant and the secondary fixation represented by osseointegration were complicated. Therefore, we must consider the complex reactions in living organisms that differ from those of the materials, including lumber and steel [17].
While the lumber and steel were stable, the alveolar bone around the screwed implant body was biologically altered. These alterations require an appropriate speed of bone remodelling. Based on the study, it is suggested that the interactions of secreted EphrinB2 and membrane-type EphB4 may have the function of avoiding abrupt changes in the living body caused by sudden force loading such as torques during implant replacement. In the future, further studies on the roles of ephrin and Eph family members other than EphrinB2 and EphB4 in the initial reactions of alveolar bone around dental implants are warranted.
Conclusion
Cyclical compressive stress and extensive stress inhibited the differentiation of MC3T3-E1 cells and altered their secreted and membrane-type EphrinB2 and EphB4 expression levels. EphrinB2- Fc-Ti restored the decreased differentiation and secreted EphrinB2 from MC3T3-E1 cells to recover the cell differentiation. Therefore, it is suggested that binding of increasedEphrinB2 secreted from the cells to increased EphB4 on the cell membrane may participate in the gradual recovery of alveolar bone damage caused by the initial compressive stress conditions during dental implant replacement.
Acknowledgement
We thank GiichiroKawachi (Kyushu University) for technical support. We also thank Alison Sherwin, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by JSPS KAKENHI Grant Number JP15K11162, JP16K11602.
References
- Hou J, Chen Y, Meng X, Shi C, Li C, Sun H, et al. Compressive force regulates EphrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement. Korean J Orthod. 2014; 44: 320-329.
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional EphrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006; 4: 111-121.
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009; 284: 14637-14644.
- Raghavendra S, Wood MC, Taylor TD. Early wound healing around endosseous implants: a review of the literature. Int J Oral Maxillofac Implants. 2005; 20: 425-431.
- Makihira S, Mine Y, Nikawa H, Shuto T, Kosaka E, Sugiyama M, et al. Immobilized-OPG-Fc on a titanium surface inhibits RANKL-dependent osteoclast differentiation in vitro. J Mater Sci Mater Med. 2010; 21: 647-653.
- Makihira S, Mine Y, Kosaka E, Nikawa H. Titanium surface roughness accelerates RANKL-dependent differentiation in the osteoclast precursor cell line, RAW264.7. Dent Mater J. 2007; 26: 739-745.
- Raab-Cullen DM, Thiede MA, Petersen DN, Kimmel DB, Recker RR. Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif Tissue Int. 1994; 55: 473-478.
- Nakao K, Goto T, Gunjigake KK, Konoo T, Kobayashi S, Yamaguchi K. Intermittent force induces high RANKL expression in human periodontal ligament cells. J Dent Res. 2007; 86: 623-628.
- Arai K, Nagashima Y, Takemoto T, Nishiyama T. Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells. Cell Struct Funct. 2008; 33: 203-210.
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983; 96: 191-198.
- Wachi T, Shuto T, Shinohara Y, Matono Y, Makihira S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015; 327: 1-9.
- Atsuta I, Yamaza T, Yoshinari M, Goto T, Kido MA, Kagiya T, et al. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat periimplant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials. 2005; 26: 6280-6287.
- Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012; 6: 148-156.
- Salvi GE, Bosshardt DD, Lang NP, Abrahamsson I, Berglundh T, Lindhe J, et al. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol 2000. 2015; 68: 135-152.
- Brunski JB. Biomechanical factors affecting the bone-dental implant interface. Clin Mater. 1992; 10: 153-201.
- Sennerby L, Roos J. Surgical determinants of clinical success of osseointegrated oral implants: a review of the literature. Int J Prosthodont. 1998; 11: 408-420.
- Mall N, Dhanasekar B, Aparna IN. Validation of implant stability: a measure of implant permanence. Indian J Dent Res. 2011; 22: 462-467.
- Atsumi M, Park SH, Wang HL. Methods used to assess implant stability: current status. Int J Oral Maxillofac Implants. 2007; 22: 743-754.
- Sartoretto SC, Alves AT, Resende RF, Calasans-Maia J, Granjeiro JM, Calasans-Maia MD. Early osseointegration driven by the surface chemistry and wettability of dental implants. J Appl Oral Sci. 2015; 23: 279-287.
- Wennerberg A, Jimbo R, Stubinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2014; 25: 1041-1050.
- Eckert SE, Meraw SJ, Cal E, Ow RK. Analysis of incidence and associated factors with fractured implants: a retrospective study. Int J Oral Maxillofac Implants. 2000; 15: 662-667.
- Ivanoff CJ, Sennerby L, Lekholm U. Influence of initial implant mobility on the integration of titanium implants. An experimental study in rabbits. Clin Oral Implants Res. 1996; 7: 120-127.
- Oshida Y, Tuna EB, Aktoren O, Gencay K. Dental implant systems. Int J Mol Sci. 2010; 11: 1580-1678.