
Research Article
Austin J Dent. 2015; 2(3): 1025.
Assessing the Effects of Different Plant Extracts on Primary Dental Plaque Colonizer and Human Fibroblast Cells
Hossam A Eid¹*, Emad A Abo-Alazm², Manea M Mosleh³, Mohammed A Alshahrani4, Tarek H Taha5, Nehal M El-Deeb6 and Gamal M Hamad7
¹Department of Oral Medicine & Periodontology, Faculty of Dentistry, Suez Canal University, Egypt and Department of Periodontics, College of Dentistry, Gulf Medical University, Ajman, UAE
²Department of Operative Dentistry, Suez Canal University, Egypt
³Department of Dentistry, Ministry of Health, Saudi Arabia
4Department of Preventive Dental Sciences, King Khalid University, Saudi Arabia
5Department of Environmental Biotechnology, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technology Applications (SRTA- CITY), New Borg El-Arab, Alexandria City, Egypt
6Department of Biopharmaceutical Product Research, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technology Applications (SRTA- CITY), New Borg El- Arab, Alexandria City, Egypt
7Department of Food Technology, Arid Land Cultivation Research Institute (ALCRI), City of Scientific Research and Technology Applications (SRTA- CITY), New Borg El-Arab, Alexandria City, Egypt
*Corresponding author: Hossam A Eid, Department of Oral medicine & Periodontology, Faculty of Dentistry, Suez Canal University, Ismailia, Egypt
Received: July 07, 2015; Accepted: September 01, 2015; Published: September 10, 2015
Abstract
The success of plant extracts in ceasing and inhibiting Streptococcus mutans (S. mutans) growth was investigated. This can provide an innovative strategy to control the progression of plaque associated diseases (periodontal diseases and dental caries) by using natural sources.
Aim of work: To screen some herbal plant extracts for their inhibitory activity on Streptococcus mutans bacterial growth, its biofilm formation ability and measuring their Minimum Inhibitory Concentration (MIC).
Methodology: This experimental study tested the antibacterial activity of fourteen prepared plant extracts against streptococcus mutans, including their Minimum Inhibitory Concentration (MIC) and inhibiting its biofilm formation using disc diffusion method with some modifications and ELISA assay plate reader respectively. Cytotoxicity assay was used for calculating the proper extract dose concentration that does not have a toxic effect on human fibroblast cells.
Results: The results showed the ability of 71.4 % of the tested extracts to cease the bacterial growth. The pattern of effectiveness can be ordered as follows: clove = pomegranate peel? sage? anise ? cardamom = thyme = myrrh ? ginger ? mint = turmeric. The recorded MIC for pomegranate peel, clove and sage was 1, 1 and 0.5 % respectively. The clove extract was able to inhibit the biofilm formation compared with pomegranate peel and sage which didn’t. The clove extract showed 78.7 % percentage of biofilim inhibition compared with positive control (S. mutans with no additions).
Keywords: Dental plaque; S. mutans; Herbal extracts; Periodontal diseases; Dental caries
Introduction
Bacterial dental plaque is described as the most complex oral biofilm and the primary initiating factor of the most prevalent oral diseases such as dental caries, periodontal diseases, and peri-implant diseases due to the microbial film formation [1,2]. Plaque accumulation due to poor oral health has been considered as possible risk factors for some chronic diseases, including gastric cancer, atherosclerosis and increased risk of preterm labor and low birth weight babies specially with chronic periodontitis particularly in developing countries [1,2]. Oral pathogens in dental plaque cause microbiological insult which disturb the immunological equilibrium leading to an abnormal host tissue response resulting in periodontal disease and dental caries [2-4]. Certain microorganisms have been associated with periodontal disease and dental caries, out of which Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg) and Streptococcus mutans (S. mutans) [4,5]. S. mutans one of the primary dental plaque colonizers which provide the aggregation of other bacteria, antimicrobial actions aiming at S. mutans would be useful to retain the progression of caries and periodontal diseases [6]. The management of periodontal disease and dental caries has been directed toward the control of plaque pathogens which involves both, the mechanical therapy and use of antimicrobial agents [7]. Commercially available medications have been tried and tested against bacterial plaque pathogens. Unfortunately, various antimicrobial drugs cannot be used safely in pregnancy with some other side effects as well as development of antibacterial resistant strains of microorganism [8]. The use of herbal products in therapeutic purposes has been accepted in several ancient and resident cultures and its therapeutic qualities are accredited. Nowadays researchers believe that herbal products cause less or no side effects compared to synthetic drugs [9]. Besides, some plants are a potential source of phytochemicals compounds with various biological including anti-inflammatory, antioxidant and antimicrobial activities and have a role in chemical control of bacterial plaque formation, hence its use in inhibiting the progression of plaque associated diseases (periodontal diseases and dental caries) is promising [9,10]. It is worthy to explore herbal plant extracts for their quality, safety, toxicity, appropriate dosage to use, and efficacy which have been tested in the present study.
Methodology
Bacterial strain and preservation
Streptococcus mutans ATCC 25175, DSM No: 20523 reference strains were obtained from MERCIN, Faculty of Agriculture, Ain Shams University, Cairo, Egypt. The bacterial strain was preserved by adding 250 μl of 60% glycerol to 750 μl overnight LB culture and kept at -80oC.
Preparation of plants extracts
The suggested probable fourteen plants were purchased from local herbal market in Alexandria, Egypt and were submitted to the standard extraction procedures according to (Wendakoon, et al.) [11] with some modifications. The obtained plants were washed three times using tape water, dried and 10 grams of each plant were submitted to extraction using 100 ml of ethanol/water (3:1 v/v). The plant/solvent mixtures were shook at 200 rpm and 30°C for 18 hr. The mixtures were then spin down at 3000 rpm for 30 min and the obtained supernatants were evaporated at 50°C for 3 days. The obtained pellets were weighed and dissolved in sterile distilled water and kept at 4°C till use.
Antibacterial activity against Streptococcus mutans
The antibacterial activity of the fourteen prepared plant extracts were tested for their antibacterial activity against streptococcus mutans using disc diffusion method according to (Duraipandiyan and Ignacimuthu, 2007) [12] with some modifications. LB agar media was prepared according to the manufacture instructions (Oxoid, England), sterilized and poured into sterile Petri plates. Overnight culture (18 h) of Streptococcus mutans was freshly prepared using sterile LB broth. The bacterial strain was diluted with sterile saline solution to reach 0.5 McFarland standard and was then spread over the LB plates using sterile cotton swabs. After dryness of the plate’s surfaces, sterile filter paper discs were added to the plates where 25 μl of each individual plant extract were loaded to each separate filter disc and the plates were then kept at 4°C for 30 min. The plates were then incubated at 30°C for 18 hr and were checked for clear zones formation. The formed clear zones were recorded and measured in millimeter.
Determination of Minimum Inhibitory Concentration (MIC)
The best three extracts that showed high antibacterial activity against Streptococcus mutans were chosen and their Minimum Inhibitory Concentration (MIC) was determined using descending concentrations of the each extract. The MIC of the three plant extracts were diluted using sterile saline and were tested for their antibacterial activity against S. mutans according to (Miri, et al.) [13] with some modifications. The different prepared concentrations were tested against the bacterial strain using disc diffusion assay as previously mentioned. The formed clear zones were measured and recorded and the MIC for each extract was determined.
Cytotoxicity assay
Cytotoxicity assay was used for determination of the treatment concentration that does not have a toxic effect on normal cells. In this assay, human fibroblast cells were used as a normal cell modeling, a cell suspension of 6×104 cell/ml was collected and seeded in 96-well plates (100 μl cell suspension per well). The plates were incubated at 37°C in humidified 5% CO2 for 24 hr. After obtaining a semi confluent cell layer, about 100 μl of different treatment concentrations were incubated with cells at the previously described conditions for 3 days. After incubation, 100 μl of neutral red stain was added to each well (Borenfreund and Puerner 1985) [14], only living cells are permeable to neutral red and incorporated it into liposomes providing a quantitative assay to the cytotoxic effects. The stain intensity was assayed using automated ELIZA microplate reader adjusted at 540 nm (reference filters 620 nm).
Quantitative assay of biofilm inhibition
The ability of the plants extracts to inhibit biofilm formation of S. mutans was determined according to El-Adawi [15] with some modifications. In brief, triplicates of 100μL of a previously prepared overnight bacterial culture in Luria broth was inoculated in 96-well flat-bottom microtiter polystyrene plate with 50μL of the nontoxic dose of the treatments. The plate was incubated for 48 hr at 30°C without shaking. The plate was decanted once and followed by washing for three times with 200 μL sterile PBS buffer. The plate was then dried at 60°C for 1 h. The remaining biofilm was stained with 0.1% crystal violet for 15 min, decanted and washed three times with 200μLof sterile distilled water. The plate was air dried for 15 min followed by addition of 150 μL of 95% ethanol. The absorbance was measured at 570 nm using ELISA assay plate reader. Streptococcus mutans ATCC 25175strain was used as the positive control and uninoculated LB broth as negative control.
DNA fragmentation assay
The ability of tested plant extracts to cause DNA fragmentation for the tested pathogen S. mutans; was detected according to Ahsan, et al. [16], with some modifications. The reported MIC for each plant was added to 10 ml LB broth containing S. mutans (106cells/ ml) and was incubated at 30°C with 200 rpm shaking for different time intervals. After 0, 1, 2 and 3 hours, one ml of each plant-bacteria combination was withdrawn and submitted to centrifugation for 5 min at 10000 rpm followed by DNA extraction (Thermo Scientific, England) according to the manufacture instructions. For control, genomic DNA was isolated from S. mutans that was cultured without plants extract. The extracted DNA was checked for fragmentation using gel documentation system (SYN GEN, USA).
Anti-inflammatory activity
Peripheral Blood Mononuclear Cells (PBMC) was chosen for this study in preparing the inflammatory modeling. PBMC are the primary source of lymphoid cells that have been reported to produce proinflammatory cytokines (IL-1, IL-6, and TNF) and chemokines (IL-8 and MCP-1) in response to Lipopolysaccharide (LPS) stimulation. Its use is facilitated by Ficoll-Hypaque density gradient centrifugation (Berthold, 1981) [17]. PBMC Cells [2 ×105] were cultured in RPMI medium supplemented with 2 mM L-glutamine, 10% Fetal Calf Serum (FCS) and 1% penicillin-streptomycin solution in flat bottom 96 well plates, incubated at 37 °C in a humidified atmosphere of 5% CO2, 95% air for 24 hr prior to stimulation with E. coli LPS; 10 μg /ml. That stimulation was carried out in the presence and absence of plant extracts and left for 17 hr in serum free RPMI medium at the previous conditions. After incubation, supernatants were harvested and stored frozen at -20°C until analysis. The concentrations of TNF-a in the supernatants of RAW 264.7 cell cultures were determined using an ELISA kit, according to the manufacturer’s instructions (BioLegend, Inc.).
Results
Antibacterial activity against S. mutans
The prepared fourteen plant extracts were tested against S. mutans for their antibacterial activity. The results showed the ability of 71.4 % of the tested extracts to cease the bacterial growth. As shown in (Table 1), about only four of the tested extracts were not able to affect the bacterial growth while ten of them were able to strongly stop its growth with different potency. The pattern of effectiveness can be ordered as follows: clove = pomegranate peel > sage > anise > cardamom = thyme = myrrh > ginger > mint = turmeric while the rest four extracts including cinnamon, nigella sativa, cress-cresson and black pepper showed no remarkable antibacterial effect against S. mutans. As shown in (Table 1), both of clove and pomegranate peel were able to record 25 mm clear zones as the potent two extracts compared with 9 mm for both of mint and turmeric extracts as the lowest ones. The recorded clear zones can be easily showed as depicted in (Figure 1).
No.
Plant Extract
Concentration %
Clear Zone (mm)
1
Mint
22.5
9
2
Cinnamon
10
0
3
Nigella sativa
5
0
4
Pomegranate peel
17.5
25
5
Ginger
17.5
10
6
Cardamom
12.5
11
7
Sage
17.5
16
8
Thyme
22.5
11
9
Anise
22.5
13
10
Cress-cresson
10
0
11
Myrrh
42.5
11
12
Turmeric
15
9
13
Black pepper
7.5
0
14
Clove
17.5
25
Table 1: Antibacterial activity of fourteen plant extract against S. mutans.
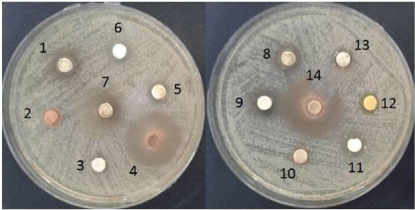
Figure 1: Clear zones formed by the effect of plants extract against S.
mutans. 1: mint, 2: cinnamon, 3: Nigella sativa, 4: pomegranate peel, 5:
ginger, 6: Cardamom, 7: sage, 8: thyme, 9: anise, 10: cress-cresson11:
myrrh, 12: turmeric, 13: black pepper, 14: clove.
Determination of MIC
The highest effective extracts were chosen as the selected extracts to pass through the rest of the work. According to this concept; pomegranate peel, clove and sage were selected and hence submitted to MIC determination process. Different dilutions of each extract were prepared and examined against the pathogenic bacteria. The data shown in (Table 2) can easily explain the used dilutions and the equivalent clear zones and so the MIC for each extract. The obtained data showed that the recorded MIC for pomegranate peel, clove and sage was 1, 1 and 0.5 % respectively. It worth to mention that; the gradual decrease in each extract concentration was matched with the observed decrease in clear zones as shown in (Figure 2).
No.
Plant Extract
Concentration %
Clear Zone (mm)
1
Pomegranate peel
17.5
24
8.7
20
4.2
16
2
13
1
10
0.5
0
2
Clove
17.5
25
8.7
20
4.2
12
2
10
1
9
0.5
0
3
Sage
17.5
23
9.5
18
4.7
15
2.25
13
1
13
0.5
10
0.3
9
0.15
0
Table 2: MIC of the tested extracts of pomegranate peel, clove and sage against S. mutans.
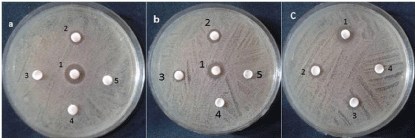
Figure 2: MIC of the selected three plant extracts against S. mutans.
(a: pomegranate peel, 1: 2%, 2: 1%, 3: 0.5%, 4: 0.25% and 5: 0.125%
respectively)(b: clove, 1: 2%, 2: 1%, 3: 0.5%, 4: 0.25% and 5: 0.125%
respectively)(c: sage, 1: 1%, 2: 0.5%, 3: 0.25% and 4: 0.125% respectively).
Cytotoxicity assay
The Cytotoxicity results of pomegranate peel, clove and sage on Human Fibroblast cells indicated that, generally, the extracts of both clove and sage was safer on cells than that of pomegranate peel (Figure 3). Pomegranate peel extract IC50 value on Fibroblast cells recorded 10 mg/ml with inhibition percentage 50.72. While, the maximum used concentrations of both clove and sage (42 and 47 mg/ ml, respectively) were not reach the IC50 on cells (Figures 4 and 5).
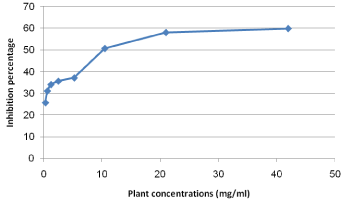
Figure 3: Cytotoxicity percentage of pomegranate peel plant extract on
fibroblast cells.
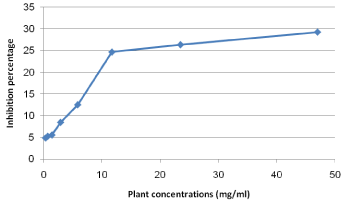
Figure 4: Cytotoxicity percentage of sage plant extract on fibroblast cells.
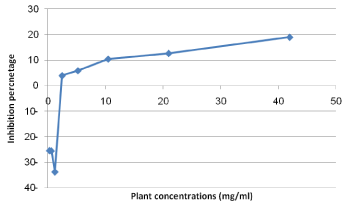
Figure 5: Cytotoxicity percentage of clove plant extract on fibroblast cells.
Biofilm inhibition
The MIC of the selected three plants extracts; pomegranate peel, clove and sage were tested for their ability to inhibit the biofilm formation of S. mutans. The obtained results revealed the success of clove extract to inhibit the biofilm formation compared with pomegranate peel and sage which didn’t (Figure 6). The clove extract showed 78.7 % percentage of biofilim inhibition compared with positive control (S. mutans with no additions).
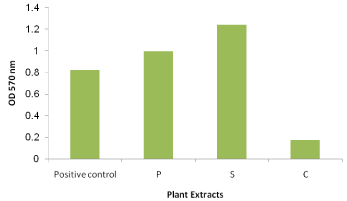
Figure 6: Biofilm inhibition of S. mutans by the reported MIC of the three
tested extracts. P: pomegranate peel, S: sage and C: clove.
DNA fragmentation
DNA fragmentation is a technique that can detect the ability of specific individual compound or mixture of compounds to degrade an intact molecule of DNA into many smaller parts. The obtained results revealed that; the three studied plant extracts (pomegranate peel, sage and clove) failed to degrade S. mutans DNA into smaller parts. It worth to mention that; the used extracts concentrations were the same as the reported MIC.
Anti-inflammatory assay
The anti-inflammatory results clarified that, all the used plant extracts (pomegranate peel, clove and sage) co-treatment could reduce TNF-a secretion in LPS-stimulated model (Figure 7) with inhibition percentage ranged from 47.9 to 65.652. The maximum TNF-a inhibition percentage recorded in cells treated with both sage and pomegranate peel plant extract with percentages 65.652 and 65.507, respectively.
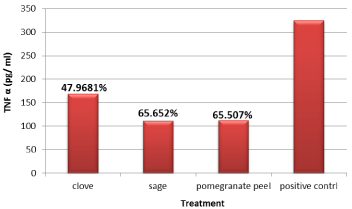
Figure 7: Anti-inflammatory effect of clove, sage and pomegranate peel.
Discussion
Periodontal disease and dental caries are among the most common diseases in affecting mankind since early history of ancient civilizations [18,19]. Since ancient times, medicinal plants have played a critical role in maintaining mankind health. The use of medicinal plants and its bioactive compounds has increased in rural areas and developing countries due to poverty, and an increased demand of inexpensive medicines [20]. Recently, researchers have developed a greater interest of using these compounds in formulation of new and novel preparations, because of their biological activities and reliability [20,21]. These biological and antimicrobial activities can be used in the chemical control of bacterial plaque formation and inhibiting the progression of plaque associated diseases (periodontal diseases and dental caries) successfully [20]. Replacing the commercially available chloride, chlorhexidine, fluoride, and fluoride-containing agents with ethanol (its active ingredient) which can cause many side effects starting from tooth staining, ending by oral cancer and teratogensis [21,22]. However, A major concern about bioactive compounds from plants is that some of these compounds are toxic to our normal cells; therefore safety is critical in development of novel drugs which were tested in this study [20,23]. The results of the present study revealed that, the antibacterial activity of the fourteen prepared plant extracts were tested for their antibacterial activity against streptococcus mutans (S. mutans). As shown in ( Table 1), both of clove and pomegranate peel were able to record 25 mm clear zones as the potent two extracts compared with 9 mm for both of mint and turmeric extracts as the lowest ones. While the rest four extracts including cinnamon, Nigella sativa, cress-cresson and black pepper showed no remarkable antibacterial effect against S. mutans. The MIC of the selected three plants extracts; pomegranate peel, clove and sage were tested for their ability to inhibit the biofilm formation of S. mutans. The obtained results revealed the success of clove extract to inhibit the biofilm formation compared with pomegranate peel and sage which didn’t (Figure 6). The clove extract showed 78.7 % percentage of biofilim inhibition compared with positive control (S. mutans with no additions). Pomegranate peel, sage and clove with the same extract concentration used in MIC failed to degrade S. mutans DNA into smaller parts. This failure may be attributed to two reasons; the first one is the used concentrations and the second is the time of exposure. We can predict that much higher concentrations are able to cause DNA fragmentation in addition to the longevity of the exposure duration. The anti-inflammatory results clarified that, all the used plant extracts (pomegranate peel, clove and sage) co-treatment could reduce TNF-a secretion in LPS-stimulated model (Figure 7) with inhibition percentage ranged from 47.9 to 65.652. The maximum TNF-a inhibition percentage recorded in cells treated with both sage and pomegranate peel plant extract with percentages 65.652 and 65.507, respectively. The Cytotoxicity results of pomegranate peel, clove and sage on human fibroblast cells indicated that, generally, the extracts of both clove and sage were safer on cells than that of pomegranate peel (Figure 3). Pomegranate peels extract IC50 value on fibroblast cells recorded 10 mg/ml with inhibition percentage 50.72. While, the maximum used concentrations of both clove and sage (42 and 47 mg/ml, respectively) were not reach the IC50 on cells (Figures 4 and 5). The obtained data revealed that the MIC of the three plant extracts can strongly cease the S. mutans growth with simultaneous safe effect on the human cells. Based on our study results, further investigations including purification and formulation of the extract are needed to develop an antibacterial mouth wash/ rinse which has anti-plaque activity that could be used safely specially in pregnant women with poor oral hygiene. The search for natural herbal products as viable alternatives to synthetic products should be continued.
Conclusion
The results of the present study showed that some plant extracts has antibacterial activity and biofilm inhibitory effects against S. mutans, besides it has anti-inflammatory activity without harmful effect on human fibroblast cells. However, more clinical trials are needed in order to emphasis this hypothesis.
Clinical Implications
Some of plant extracts could be used as chemical anti-plaque and anti-cariogenic agents besides, its anti-inflammatory effect in the field of dentistry with minimal or no risk of development of bacterial resistance strains.
References
- Gunsolley JC. Clinical efficacy of antimicrobial mouth rinses. J Dent. 2010; 38: 6-10.
- Pihlstrom BL. Periodontology for the General Practitioner. Periodontal 2000. 2001; 25: 1-25.
- Socransky SS, Haffajee AD. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: A critical assessment. J Periodontal Res. 1991; 26: 195-212.
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994; 8: 263-271.
- Pavan R, Jain S, Shraddha, Kumar A. Properties and therapeutic application of bromelain: A review. Biotechnol Res Int. 2012; 12: 976-983.
- Formagio AS, Kassuya CA, Neto FF, Volobuff CR, Iriguchi EK, Maria do C Vieira, et al. The flavonoid content and antiproliferative, hypoglcaemic, anti-inflammatory and free readicals scavenging activities of Annona dioica St. Hill. BMC Complement Altern Med activities of Annona dioica St. Hill. BMC Complement Altern Med. 2013; 13: 14-19.
- Khosropanah H, Bazargani A, Ebrahimi H, Eftekhar K, Emami Z, Esmailzadeh S. Assessing the effect of pineapple extract alone and in combination with vancomycin on Streptococcus sanguis. Jundishapur J Nat Pharm Prod. 2012; 7: 140-143. 8.
- NC Praveen, A Rajesh, Manish Madan, Vishwajit Rampratap Chaurasia, Neel V Hiremath, Akanksha Manmohan Sharma. In vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. Journal of International Oral Health. 2014; 6: 96-98. 9.
- Afolayan AJ. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bac-teria and fungi. Pharm Biol. 2003; 41: 22-25.
- Gottlieb OR, Borin MR, Brito NR. Integra-tion of ethnobotany and phytochemistry: dream or reality? Phytochemistry. 2002; 60: 145-15.
- Wendakoon C, Calderon P, Gagnon D. Evaluation of Selected Medicinal Plants Extracted in Different Ethanol Concentrations for Antibacterial Activity against Human Pathogens. Journal of Medicinally Active Plants. 2012; 1: 60-68.
- Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Cassia fistula L.: an ethnomedicinalplant,” Journal of Ethnopharmacology. 2007; 112: 590-594.
- Miri A, Rad JS, Alfatemi SMH, Rad MS. A study of Antibacterial potentiality of some plants extracts against multidrug resistant human pathogens. Annals of Biological Research. 2013; 4: 35-41.
- Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985; 24: 119-124.
- Aladawi H. Inhibitory effect of Grape Seed Extract (GSE) on cariogenic bacteria. Journal of Medicinal Plants Research. 2012; 6: 4883-4891.
- Ahsan N, Paul N, Islam N, Akhand AA. Leaf Extract of Syzygiumcumini Shows Anti-Vibrio Activity Involving DNA Damage. Dhaka Univ J Pharm Sci. 2012; 11: 25-28.
- Berthold F. Isolation of human monocytes by ficoll density gradient centrifugation. Blut. 1982; 43: 367-371.
- Judit Forrai . The beginnings of dental caries and its treatments. Rev Clín Pesq Odontol. 2009; 5: 187-192.
- Forrai J. Culture history of dentistry. Dental Press: Budapest. 2005.
- Shai LJ, McGaw LJ, Masoko P, Eloff JN. Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans. South African Journal of Botany. 2008; 74: 677-684.
- Taur DJ, Waghmare MG, Bandal RS, Patil RY. Antinociceptive activity of Ricinus communis L. leaves. Asian Pacific Journal of Tropical Biomedicine. 2011; 1: 139-141.
- Subramaniam P, Eswara U, Maheshwar Reddy KR. Effect of different types of tea on Streptococcus mutans: An in vitro study. Indian J Dent Res. 2012; 23: 43-48
- McCullough MJ, Farah CS. The role of alcohol in oral carcinogenesis with particular reference to alcohol containing mouth washes. Aust Dent J. 2008; 53: 302-305.