
Opinion
Austin Diabetes Res. 2016; 1(1): 1004.
Central and Peripheral Taurine Levels in Diabetic Rats under Depressive-Like Behavior Treated with Insulin and/or Clonazepam
Wayhs CAY¹*, Tortato C¹, Mescka CP¹, Sitta A³, Ribas GS4, Guerreiro G¹, Barros HMT5 and Vargas CR1,3,6*
¹Pharmaceutical Sciences Graduate Program, Federal University of Rio Grande do Sul, Brazil
²Pharmacy Service, Clinic Hospital of Porto Alegre, Brazil
³Medical Genetics Service, Clinic Hospital of Porto Alegre, Brazil
4Graduate Program in Child and Adolescent Health, Federal University of Rio Grande do Sul, Brazil
5Department of Basic Health Sciences, Federal University of Health Sciences of Porto Alegre, Brazil
6Graduate Program in Biological Sciences: Biochemistry, Federal University of Rio Grande do Sul, Brazil
*Corresponding author: Wayhs CAY and Vargas CR, Pharmaceutical Sciences Graduate Program, Federal University of Rio Grande do Sul, Brazil
Received: July 22, 2016; Accepted: August 03, 2016; Published: August 05, 2016
Abstract
Taurine (2-aminoethanesulfonic acid) is a sulfur-containing amino acid that is involved in a variety of physiological functions. Considering that many reports indicate that taurine participates in the development of diabetes and also appears to play a role in the pathophysiology of depression, the aim of this study is to highlight the insulin and/or clonazepam effect on plasma and cerebral cortex taurine concentrations of diabetic rats submitted to forced swimming test. Previous studies of our group showed that diabetic rats present depressive-like behavior and oxidative damage to biomolecules and that the association of insulin plus clonazepam is able to reverse this process. In the present study, it was verified a longer immobility time in diabetic rats, which was prevented by insulin plus clonazepam acute treatment. Moreover, taurine concentrations were decreased in plasma and increased in cerebral cortex from the rats, demonstrating that in this experimental animal model of diabetes and depression occurs a deficiency of this important amino acid in plasma, as well as a high uptake by the brain. It was also observed that these effects were corrected by the insulin and/or clonazepam acute treatment, suggesting that this therapeutic association is important to restore taurine homeostasis in diabetic rats under depressive-like behavior.
Keywords: Diabetes; Depression; Oxidative stress; GABA agonist; Osmoregulation; Cerebral edema
Introduction
Taurine, a 2-aminoethanesulfonic acid, is one of the most abundant free amino acid in the central nervous system and in the peripheral tissues [1], accounting for approximately 0.1% of total human body weight [2]. The main source of taurine in humans is the diet and the rate of endogenous synthesis is relatively low [3]. The physiological and therapeutic properties of this amino acid have been studied. Taurine modulates a variety of fundamental biological functions, including anti-oxidation, Ca2+ transport regulation, antiinflammation, osmoregulation [2,4], anti-obesity action [5], neuronal modulation, protection against oxidative stress [6] and hypoglycemic action [7-9].
Several studies indicate that taurine participates in the development of diabetes, since its plasma concentrations are found to be low in these patients [10,11], suggesting that diabetes can be considered a taurine-deficient condition [2]. Moreover, this amino acid is involved in mental disorders such as depression, since it was demonstrated that taurine is greatly diminished in plasma and cerebrospinal fluid of depressive patients [12] and it was verified that its supplementation had an antidepressant effect in diabetic rats exposed to Forced Swimming Test (FST) [13]. In fact, there is a well-known link between depression and diabetes, since studies have shown that diabetic individuals present more depressive behaviors that non-diabetic individual [14-18].
Evidence suggest that Gamma-Amino Butyric Acid (GABA) neurotransmitter plays a role in the pathophysiology of depression, since GABA agonists, like clonazepam, have been prescribed as adjuvant for the treatment of depression in humans [19,20]. In this context, taurine acts as an agonist at inhibitory GABA subtype a receptors (GABAA) [1] and its supplementation modulates glucose homeostasis and regulates insulin release from pancreatic beta cells, improving the glycemic profile in diabetic individuals [21-24].
Preclinical studies have also shown that diabetic rats and mice have more depressive-like behaviors than non-diabetic animals in the Forced Swimming Test (FST), since the duration of immobility time is longer in diabetic when compared to nondiabetic animals in this experimental animal model of depression [16,25]. Insulin plus clonazepam treatment reversed the prolonged immobility in diabetic rats [26]. Furthermore, it was verified that the association of insulin plus clonazepam in an acute administration was able to partially reverse this effect [27]. Considering that many reports indicate that taurine participates in the development of diabetes and also appears to play a role in the pathophysiology of depression, the purpose of this study is to investigate the insulin and/or clonazepam effect on plasma and cerebral cortex taurine concentrations of diabetic rats under depressive-like behavior.
Materials and Methods
Animals
Male Wistar adult rats (250-300 g), born and reared in the animal facility of Universidade Federal de Ciencias da Saude de Porto Alegre (UFCSPA), Brazil, were housed in polypropylene cages (40x33x17 cm), four per cage, under standard environmental conditions, such as a room temperature of 22±2°C and a 12 h light-dark cycle (7:00 a.m.- 7:00 p.m.). All rats had free access to food and water. The animals were divided into five groups: controls (nondiabetic); diabetics submitted to FST (STZ+FST); diabetics submitted to FST treated with insulin (STZ+FST–INS); diabetics submitted to FST treated with clonazepam (STZ+FST–CNZ); and diabetics submitted to FST treated with insulin plus clonazepam (STZ+FST–INS+CNZ). All groups were submitted to FST plus Streptozotocin (STZ), except control group that was not submitted to STZ. Our experimental protocol was carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and in accordance with the Brazilian Law for the Scientific Use of Animals after its approval by the Ethical Committee for Animal Experimentation at UFCSPA (050/11). All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data.
Drugs
Clonazepam (0.25 mg/mL; Rivotril®, Roche, Brazil) and streptozotocin (60 mg/mL; Sigma, St. Louis, MO, USA) was prepared in citrate buffer (pH 4.3). Insulin (dose, 4 IU/mL) was administered intraperitoneally (i.p.) (Humulin®, Lilly, USA). It should be noted that prior to the experiment it was conducted a pilot study with the insulin dose cited to verify its efficacy in this model and avoid the risk of hypoglycemia in the animals. All solutions were prepared immediately before i.p. administration.
Diabetes induction
Nondiabetic control rats received i.p. injections of saline (1 mL/kg) and were also submitted to blood glucose measurement to confirm that they presented normal blood glucose levels. Diabetes was induced by a single i.p. dose of STZ, 60 mg/kg, as described previously [16]. Increased blood glucose levels (≥13.875 mM) of STZ-rats (blood collected from tail) were confirmed with a glucometer (AccuChek Active®, Roche, Germany) after 72h. All animals became diabetics.
Forced swimming test (FST)
After 21 days of diabetes induction, animals were submitted to the FST [28]. On the first day of the experiment (training session), 24h before the FST, the animals were placed in the aquarium for 15 min (22×22×35 cm) with water level of 27 cm and water temperature of 25+2°C. Soon after, the rats were gently dried with towels and the first drug dose was administered i.p. (insulin 4 IU/kg, clonazepam 0.25 mg/kg i.p., insulin 4 IU/kg+clonazepam 0.25 mg/kg or 1 mL/ kg saline). The FST session was performed after 24h, in the same conditions described above, lasting for 5 minutes. The animals received additional dosing of their respective treatments 5 and 1h before being submitted to the FST. Behaviors in the test session were recorded for subsequent ethological analysis by a trained researcher who was blind to the different treatments (BASIC software, Kevin Willioma, KD Ware Computer, Boston, MA). Immobility was defined as the sum of the freezing and floating behaviors. The antidepressant effect of the drugs was inferred when they decreased immobility duration behaviors. All behavioral experiments were performed between 1:00 and 5:00 p.m. It is important to note that a control group was added in the FST to elucidate the behavioral changes of diabetic animals.
Brain micro dissection and tissue preparation
Thirty minutes after the FST, the animals were sacrificed by decapitation and brains were immediately removed and kept on an ice-plate. Cerebral cortex were dissected and kept chilled until homogenization. The cerebral cortexes were homogenized 1:10 w/v in 20 mM sodium phosphate and 140 mM KCl (pH 7.4) buffer. Homogenates were centrifuged at 750g for 10 min at 4°C and the supernatant was immediately used for measurements.
Taurine determination
The free amino acid taurine in plasma was determined by HPLC method [29], using fluorescence detection. Taurine was quantitatively determined by relating its chromatographic peak area with those obtained from a known standard mixture and with the internal standard peak area (homocysteic acid). The results were expressed as Umol/L.
Statistical analyses
Statistical analyses were performed using independent-samples T test and one-way analysis of variance (ANOVA), followed by the Duncan multiple range test when appropriate. The Pearson correlation test was used to evaluate the correlation between the biochemical variables. Figures data were expressed as mean±standard error of mean (SEM) and table data were expressed as Mean±Standard Deviation (SD). All analyses were performed using the Statistical Package for the Social Sciences (SPSS 14.0 for Windows Evaluation Version) software. A P value < 0.05 was considered as statistically significant difference.
Results
Glycemia of animals from the different groups after FST and before the decapitation is shown in Table 1. It can be observed that isolated insulin or insulin plus clonazepam acute treatment significantly decreased glycemia when compared to non treated diabetic rats (STZ) [F(4,42)=58.539 P<0.001]. As expected, clonazepam treatment did not modify the diabetic rat’s glycemia.
Groups
no of contents
Blood glucose levels (mM)
(mean ± SD)
Control
9
5.91 ± 0.94
STZ+FST
10
29.22 ± 3.59*
STZ+FST-INS
10
19.66 ± 4.61*#
STZ+FST-CNZ
8
29.37 ± 3.38*
STZ+FST-INS+CNZ
10
22.47 ± 4.72*#
SD: Standard Deviation; STZ: Streptozotocin; INS: Insulin; CNZ: Clonazepam; * P<0.05 compared to the control; # P<0.05 compared to STZ+FST group (ANOVA followed by DUNCAN test).
Table 1: Blood glucose levels 30 min after forced swimming test and before the decapitation from streptozotocin-induced diabetic rats submitted to forced swimming test not treated (STZ+FST) and treated with insulin (STZ+FST-INS) or clonazepam (STZ+FST-CNZ) or insulin plus clonazepam (STZ+FST-INS+CNZ) (n=8–12) and controls (n=9). Data represent mean ± S.D. * p<0.05 compared to the control group (ANOVA followed by DUNCAN test).
Table 2 shows body weight soon before forced swimming test training session and 24 hours water consumption after 6 and 11 days of diabetes induction from streptozotocin-induced diabetic rats and controls. It can be seen that diabetic animals lost weight during the 21 days of induction of diabetes and increased daily water consumption, which classically occurs in an experimental model of streptozotocininduced diabetes (p<0.05).
Groups
No of Rats
Body weight (g)
(mean ± SD)
24h water consumption (mL/cage) after 6 days of STZ
(mean ± SD)
24h water consumption (mL/cage) after 11 days of STZ (mean ± SD)
Control
9
274.44 ± 25.79
200.00 ± 70.71
175.00 ± 35.35
Diabetic rats (STZ)
38
248.16 ± 21.23*
600.00 ± 100.00*
612.50 ± 118.94*
SD: Standard Deviation; STZ: Streptozotocin; * P<0.05 compared to the control (Independent-samples T Test).
Table 2: Body weight soon before forced swimming test training session and 24h water consumption after 6 and 11 days of diabetes induction from streptozotocininduced diabetic rats (STZ) (n=38) and controls (n=9). Data represent mean ± S.D. * p<0.05 compared to the control group (Independent-samples T Test).
Figure 1 shows the immobility time of STZ rats submitted to FST treated or not treated with insulin and/or clonazepam and controls. It is possible to observe that STZ-induced diabetic rats presented longer immobility time when compared to control group [F(4,42)=3.183 P<0.05]. Moreover, the association of insulin plus clonazepam acute treatment was capable to reverse this depressive-like behavior.
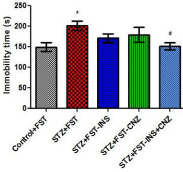
Figure 1: Immobility time from streptozotocin-induced diabetic rats submitted
to the forced swimming test not treated (STZ+FST) or treated with insulin
(STZ+FST–INS), clonazepam (STZ+FST–CNZ) or insulin + clonazepam
(STZ+FST–INS–CNZ) (n=9–10) and controls (Control+FST) (n=9). Data
represent mean ± SEM * p<0.05 compared to the control groups; # p < 0.05
compared to the STZ group (ANOVA followed by DUNCAN Test).
Plasma taurine levels from animals treated or not with insulin and/or clonazepam are presented in Figure 2. It can be verified that plasma taurine concentrations were significantly decreased in STZ groups treated or not with insulin or clonazepam when compared to control group. Furthermore, plasma taurine concentration was reverted to control levels by insulin plus clonazepam acute treatment [F(4,42)=3.775 P<0.05].
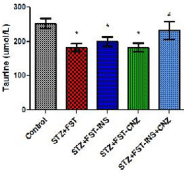
Figure 2: Taurine level in plasma from streptozotocin-induced diabetic rats
submitted to the forced swimming test not treated (STZ+FST) or treated
with insulin (STZ+FST–INS), clonazepam (STZ+FST– CNZ) or insulin +
clonazepam (STZ+FST–INS–CNZ) (n=9–11), and controls (n=8). Data
represent mean ± SEM * p<0.05 compared to the control groups; # p<0.05
compared to the STZ group (ANOVA followed by DUNCAN Test).
As shown in Figure 3, the cerebral cortex taurine levels from untreated STZ rats submitted to FST were significantly increased when compared to control group and only treatment with insulin reverted to control levels [F(4,39)=3.477 P<0.05].
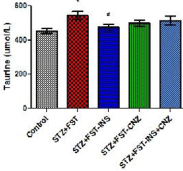
Figure 3: Taurine level in cerebral cortex from streptozotocin-induced
diabetic rats submitted to the forced swimming test not treated (STZ+FST) or
treated with insulin (STZ+FST–INS), clonazepam (STZ+FST–CNZ) or insulin
+ clonazepam (STZ+FST–INS–CNZ) (n=7–11), and controls (n=8). Data
represent mean ± SEM * p<0.05 compared to the control groups; # p<0.05
compared to the STZ group (ANOVA followed by DUNCAN Test).
It is noteworthy that a significant positive correlation between glycemia and the immobility time (r=0.308; P<0.05) and between glycemia and cerebral cortex taurine levels (r=0.377; P<0.05) were observed in treated and untreated STZ-rats submitted to FST. Moreover, a negative correlation between glycemia and plasma taurine levels (r=-0.440; P<0.01) and also between plasma and cerebral cortex taurine levels (r=-0.344; P<0.05) were observed in treated and untreated STZ-rats submitted to FST. However, the correlation between glycemia and taurine in both plasma and cortex is not strong.
Discussion
Considering the higher depressive-like behavior in diabetic mice and rats submitted to the forced swimming test and the beneficial effects of insulin and/or clonazepam treatment on behavioral changes and oxidative stress parameters in plasma [27,30], liver [31] and brain [18,32] of these animals, our goal in this study was to investigate the effect of these drugs on plasma and cerebral cortex taurine concentrations in diabetic rats under depressive-like behavior, since this amino acid has shown to exert hypoglycemic and antidepressant actions in previous studies.
Therefore, it was determined the glycemia status and the immobility time from the animals in order to evidence the experimental animal model of diabetes under depressive-like behavior. Our findings showed that STZ-induced diabetic rats presented significantly increased glycemia and longer immobility time. Treatment with insulin plus clonazepam reversed the immobility time in FST, providing evidence of an antidepressant effect of this association in this experimental animal model, which was not evidenced by only insulin or clonazepam treatments.
The hypoglycemic effects of taurine in plasma from diabetic animal models and in humans have been intensively studied in the last few decades, but underlying mechanisms have not been still totally elucidated. Multiple mechanisms are reported to be involved: improvement of insulin sensitivity [1,33]; stimulation of insulin secretion (by up regulating the expression levels of genes involved in the secretion of insulin and/or by inhibiting ATP-sensitive K+ channels) [23,34]; anti-oxidation (by protecting the mitochondrial excessive superoxide generation through the conjugation with the key uridine moiety of mitochondrial 5-taurinomethyluridine) [35]; and anti-inflammation [36,37].
Moreover, another mechanisms are also described, like antioxidation (by protecting the mitochondrial excessive superoxide generation through the conjugation with the key uridine moiety of mitochondrial 5-taurinomethyluridine (tRNALeu) [35]; and anti-inflammation (by suppressing the secretion of diabetes related cytokines including Tumor Necrosis Factor (TNF-α) and Monocyte Chemotactic Protein (MCP-1) [36,37]. It has been shown that taurine supplementation modulates glucose homeostasis and regulates insulin release from pancreatic beta cells, improving the glycemic profile in diabetic individuals [22-24].
It was verified a significant decrease of plasma taurine levels in diabetic rats submitted to FST, as well as a negative correlation between glycemia and plasma taurine levels. Our results are in agreement with the literature, suggesting that diabetes and depressivelike behavior status is really a plasma taurine-deficient condition [13]. Taurine deficiency in plasma from diabetic patients can be explained by the low intestinal absorption rates and high renal excretion rates of taurine in these patients [6]. In addition, declines of this amino acid levels are observed in the liver of diabetic animals [38]. Moreover, it was evidenced that the activities of taurine transporters are inhibited in high glucose conditions [39] and demonstrated that its intracellular concentration was depleted in response to the intracellular accumulation of sorbitol [21]. Therefore, the bioavailability of taurine is low in patients with diabetes and taurine deficiency may be one reason of diabetes development [2].
Our results also showed that the associated treatment of insulin plus clonazepam reversed plasma taurine concentrations to control levels, having a protecting action upon this process. One hypothesis to explain this effect is that the association insulin plus clonazepam can be acting as an antioxidant treatment in this experimental model, what could contribute to maintain plasma taurine at levels similar to controls, since taurine is an important antioxidant. In previous studies published by our research group, treatment with insulin plus clonazepam prevented oxidative damage in STZ rats submitted to FST [27,30].
In this study, taurine concentration was determined in cerebral cortex of the animals since taurine is considered one the most important intracellular osmolytes in the brain [40] and in the diabetic state, elevated glucose levels may disturb cellular osmoregulation [41]. It was verified that brain taurine levels were significantly increased in untreated diabetic rats submitted to the FST. Furthermore, a negative correlation between plasma and cerebral cortex taurine levels was observed in STZ-rats submitted to FST, demonstrating that in this experimental animal model of diabetes and depression occurs a deficiency of taurine in plasma which could be associated with a high brain taurine uptake.
The increased extracellular levels of glucose in diabetes represent an osmotic stress for the cells that could result in cellular dysfunctions [41] and even in diabetic cerebral edema, with death or severe neurological squeal [42]. The findings of our present study are extremely important, providing evidence the hypothesis that hyperglycemic status modify the taurine flux from brain cells, probably increasing sodium and decreasing potassium concentrations [43], which are associated with changes in the neuro-osmosregulation and hyperosmotic insults [42] or through changes in the fluidity of diabetic rat brain synaptossomal lipids [44], what will be better studied in the future.
On the other hand, it was observed that the treatment with insulin reverted brain taurine concentrations to control levels. Our results are in accordance with data from literature showing that diabetic rat’s present increased synaptossomal taurine uptake and therefore, higher brain taurine levels compared with normoglycemic control animals. Treatment with insulin was able to restore synaptossomal taurine uptake to the level observed in normoglycemic controls, probably by normalizing the serum glucose concentrations [45]. Besides, it was not observed modifications in brain taurine levels in STZ+FST rats treated with clonazepam. This finding could be explained by the fact that clonazepam does not alter blood glucose in diabetic animals, consequently not interfering in the taurine flux in brain cells and, therefore, maintaining the neuro-osmosregulation presented in the hyperglycemic rats and the high cerebral cortex taurine concentrations.
The hypothesis that the changes observed in brain and plasma taurine concentrations in this experimental animal model of diabetes and depression could be occurring as a result of GABA neurotransmission cannot be discarded. GABA cerebral levels will be measured in future to elucidate this hypothesis. Alterations in the taurine concentrations are intrinsically linked to changes in the levels of GABA, since taurine is structurally related to GABA and acts as an agonist for GABAA receptors, increasing plasma and brain GABA levels [1,10]. In this context, it is also possible to establish the hypothesis that taurine and clonazepam might be competing for the same receptor, what should be better investigated in the future. Furthermore, it is noteworthy that the endocrine pancreas is regulated by glutamate and GABA to control insulin and glucagon release through α- and β-cells [46] and the elevated glucose levels stimulate insulin and GABA release in β-cells [47,48].
In summary, our findings showed that taurine concentrations are decreased in plasma and increased in cerebral cortex in diabetic rats under depressive-like behavior, demonstrating that in this experimental animal model occurs a plasma taurine-deficient condition and a brain high taurine uptake condition, what can be implicated in the pathophysiology of diabetes and depression. Treatment with insulin and/ or clonazepam was able to correct this disbalance, contributing to restore taurine homeostasis in diabetic rats under depressive-like behavior, what can be relevant for the understanding of diabetic encephalopathy.
Acknowledgement
We thank Mario Serapiao for his technical support. We also appreciate the financial support from Fundacao de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and Fundo de Incentivo a Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA). CRV is the recipient of a CNPq 1D Researcher Productivity Grant. HMTB is the recipient of a CNPq 1C Researcher Productivity Grant.
References
- Wu JY, Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010; 17
- Imae M, Asano T, Murakami S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino acids. 2012; 46: 81-88.
- Wojcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010; 208: 19-25.
- Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010; 17.
- Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, et al. Taurine deficiency creates a vicious circle promoting obesity. Endocrinology. 2006; 147: 3276-3284.
- Merheb M, Daher RT, Nasrallah M, Sabra R, Ziyadeh FN, Barada K. Taurine intestinal absorption and renal excretion test in diabetic patients: a pilot study. Diabetes care. 2007; 30: 2652-2654.
- Ribeiro RA, Bonfleur ML, Amaral AG, Vanzela EC, Rocco SA, Boschero AC, et al. Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets. Diabetes Metab Res Rev. 2009; 25: 370-379.
- Das J, Vasan V, Sil PC. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2012; 258: 296-308.
- Elizarova EP, Nedosugova LV. First experiments in taurine administration for diabetes mellitus. The effect on erythrocyte membranes. Advances in experimental medicine and biology. 1996; 403: 583-588.
- Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, et al. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr. 1995; 61: 1115-1119.
- De Luca G, Calpona PR, Caponetti A, Romano G, Di Benedetto A, Cucinotta D, et al. Taurine and osmoregulation: platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism. 2001; 50: 60-64.
- Perry TL, Bratty PJ, Hansen S, Kennedy J, Urquhart N, Dolman CL. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch Neurol. 1975; 32: 108-113.
- Caletti G, Olguins DB, Pedrollo EF, Barros HM, Gomez R. Antidepressant effect of taurine in diabetic rats. Amino Acids. 2012; 43: 1525-1533.
- Bouwman V, Adriaanse MC, van 't Riet E, Snoek FJ, Dekker JM, Nijpels G. Depression, anxiety and glucose metabolism in the general dutch population: the new Hoorn study. PloS one. 2010; 5.
- Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005; 19: 113-122.
- Gomez R, Barros HM. Ethopharmacology of the antidepressant effect of clonazepam in diabetic rats. Pharmacol Biochem Behav. 2000; 66: 329-335.
- Yasin Wayhs CA, Tannhauser Barros HM, Vargas CR. GABAergic Modulation in Diabetic Encephalopathy-Related Depression. Curr Pharm Des. 2015; 21: 4980-4988.
- Wayhs CA, Mescka CP, Guerreiro G, Moraes TB, Jacques CE, Rosa AP, et al. Diabetic encephalopathy-related depression: experimental evidence that insulin and clonazepam restore antioxidant status in rat brain. Cell Biochem Funct. 2014; 32: 711-719.
- Morishita S. Clonazepam as a therapeutic adjunct to improve the management of depression: a brief review. Hum Psychopharmacol. 2009; 24: 191-198.
- Smith WT, Londborg PD, Glaudin V, Painter JR, Summit Research N. Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression?. J Affect Disord. 2002; 70: 251-259.
- Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001; 17: 330-346.
- Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol. 2009; 87: 91-99.
- Carneiro EM, Latorraca MQ, Araujo E, Beltra M, Oliveras MJ, Navarro M, et al. Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem. 2009; 20: 503-511.
- L'Amoreaux WJ, Cuttitta C, Santora A, Blaize JF, Tachjadi J, El Idrissi A. Taurine regulates insulin release from pancreatic beta cell lines. J Biomed Sci. 2010; 17.
- da Silva Haeser A, Sitta A, Barschak AG, Deon M, Barden AT, Schmitt GO, et al. Oxidative stress parameters in diabetic rats submitted to forced swimming test: the clonazepam effect. Brain research. 2007; 1154: 137-143.
- Hilakivi-Clarke LA, Wozniak KM, Durcan MJ, Linnoila M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol Behav. 1990; 48: 429-433.
- Wayhs CA, Manfredini V, Sitta A, Deon M, Ribas G, Vanzin C, et al. Protein and lipid oxidative damage in streptozotocin-induced diabetic rats submitted to forced swimming test: the insulin and clonazepam effect. Metab Brain Dis. 2010; 25: 297-304.
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977; 266: 730-732.
- Wajner M, Coelho DM, Barschak AG, Araujo PR, Pires RF, Lulhier FL, et al. Reduction of large neutral amino acid concentrations in plasma and CSF of patients with maple syrup urine disease during crises. J Inherit Metab Dis. 2000; 23: 505-512.
- Wayhs CA, Manfredini V, Sitta A, Deon M, Ribas GS, Vanzin CS, et al. Effects of insulin and clonazepam on DNA damage in diabetic rats submitted to the forced swimming test. Mutation Mutat Res. 2010; 703: 187-190.
- Wayhs CA, Tortato C, Mescka CP, Pasquali MA, Schnorr CE, Nin MS, et al. The association effect of insulin and clonazepam on oxidative stress in liver of an experimental animal model of diabetes and depression. Pharmaceutical biology. 2013; 51: 533-538.
- Wayhs CA, Mescka CP, Vanzin CS, Ribas GS, Guerreiro G, Nin MS, et al. Brain effect of insulin and clonazepam in diabetic rats under depressive-like behavior. Metabolic brain disease. 2013; 28: 563-570.
- Maturo J, Kulakowski EC. Taurine binding to the purified insulin receptor. Biochem Pharmacol. 1988; 37: 3755-3760.
- Park EJ, Bae JH, Kim SY, Lim JG, Baek WK, Kwon TK, et al. Inhibition of ATP-sensitive K+ channels by taurine through a benzamido-binding site on sulfonylurea receptor 1. Biochem Pharmacol. 2004; 67: 1089 -1096.
- Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino acids. 2012; 42: 2223-2232.
- Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. Amino Acids. 1993; 54: 119-124.
- Liu Y, Quinn MR. Chemokine production by rat alveolar macrophages is inhibited by taurine chloramine. Immunology letters. 2002; 80: 27-32.
- Nandhini AT, Thirunavukkarasu V, Anuradha CV. Taurine modifies insulin signaling enzymes in the fructose-fed insulin resistant rats. Diabetes Metab. 2005; 31: 337-344.
- Shi YR, Gao L, Wang SH, Bu DF, Zhang BH, Jiang HF, et al. Inhibition of taurine transport by high concentration of glucose in cultured rat cardiomyocytes. Metabolism. 2003; 52: 827-833.
- Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL. A possible role for taurine in osmoregulation within the brain. J Neurochem. 1988; 51: 740-745.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995; 333: 1260-1266.
- Koves IH, Russo VC, Higgins S, Mishra A, Pitt J, Cameron FJ, et al. An in vitro paradigm for diabetic cerebral oedema and its therapy: a critical role for taurine and water channels. Neurochemical research. 2012; 37: 182-192.
- Rose SJ, Bushi M, Nagra I, Davies WE. Taurine fluxes in insulin dependent diabetes mellitus and rehydration in streptozotocin treated rats. Adv Exp Med Biol. 2000; 483: 497-501.
- Medow MS, Kletter LB, Trachtman H. Increased lipid fluidity in synaptosomes from brains of hyperosmolal rats. Biochimica et biophysica acta. 1994; 1193: 323-329.
- Trachtman H, Futterweit S, Sturman JA. Cerebral taurine transport is increased during streptozocin-induced diabetes in rats. Diabetes. 1992; 41: 1130-1140.
- Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC. The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology. 1991; 129: 2521-2529.
- Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995; 64: 689-719.
- Tal M, Wu YJ, Leiser M, Surana M, Lodish H, Fleischer N, et al. [Val12] HRAS downregulates GLUT2 in beta cells of transgenic mice without affecting glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 1992; 89: 5744-5748.