
Review Article
J Dis Markers. 2014;1(1): 1004.
Interleukin 23 P 19 Gene Expressions in Patients with Ulcerative Colitis and its Relation to Disease Severity
Hanan El-Bassat, Lobna AboAli, Sahar El Yamany, Hanan Al Shenawy1*, Rasha A Al Din2 and Atef Taha3
1Department of Pathology, Tanta University, Egypt
2Microbiology and Immunology, Tanta University, Egypt
3Internal Medicine, Tanta University, Egypt
*Corresponding author: Hanan Al-Saeid Al-Shenawy, pathology department, Faculty of Medicine, Tanta University, 25 Hamdy Gado street, Tanta, Egypt
Received: July 09, 2014; Accepted: Aug 03, 2014; Published: Aug 07, 2014
Abstract
Background and Study Aims: The pur pose of This study Is to détermine whether mucosal expression of IL 23 p 19 has rôle in the pathogenèses of ulcerative colitis and its relation to dise ase severity.
Materials and Methods: This study was carried out on 50 patients with ulcerative colitis and 10 normal individuals as control. They were divided into: Group I: 27 patients with mild to moderate disease. Group II: 23 patients with severe disease. Group III: 10 normal individuals. All patients and control were subjected to histopathological study, IL-23p19 immunohistochemical staining, IL-23R expression by flow cytometry and serum IL-23 by ELISA.
Results: There is significant increased in IL-23p19 gene and IL-23R in ulcerative colitis patients compared with control. Significant positive correlations were detected between increased expression of IL-23 p19 gene, IL-23R, high serum IL-23 and severity of the disease.
Conclusion: increased expression of IL-23 p19 gene has a role in the pathogenesis of ulcerative colitis and that targeted therapy directed against IL- 23 p19 may be effective in the treatment. Increased expression of IL-23p19 gene and IL-23R with high serum IL-23 correlate positively with disease severity.
Keywords: Interleukin 23 p 19 gene; IL-23 R expression; Ulcerative colitis
Introduction
Inflammatory Bowel Disease (IBD) consists of two distinct diseases, Crohn's Disease (CD) and Ulcerative Colitis (UC). Both diseases are thought to arise due to combination of genetic variations and alteration in bacterial flora which can subsequently drive a deregulated immune response that results in chronic intestinal inflammation [1,2].
Interleukin 23 (IL-23) is a member of a small family of proinflammatory cytokine, consisting of a p19 subunit and a common p40 subunit [3]. The receptor for IL-23 (IL-23 R) consists of the IL-12R beta 1 subunit and a novel component termed IL-23R [4], which is expressed predominantly on T, NK, and NKT cells and to a smaller extent on monocytes, macrophages and DCs [5]. IL-23 plays a crucial role in the pathogenesis of a number of immune-mediated inflammatory diseases by recruitment of several inflammatory cells and Th17 cells [6,7]. IL-23 promotes Th17 cells producing TNF a, IL-17, IL-6, IL-22, GM-CSF, and other novel factors, which are associated with the induction of autoimmune inflammation [6,8,9]. This study was carried out to determine whether mucosal expression of IL 23 p 19 has a role in the pathogenesis of ulcerative colitis and to elucidate its relation to disease severity
Materials and Methods
Patients and methods
This study was carried out on 50 patients with ulcerative colitis and 10 individuals whose colonoscopic and histopathologic findings were normal as control. The studied subjects were selected from the inpatient and outpatient clinics of tropical medicine and internal medicine departments, Tanta University Hospital, Tanta, Egypt, in the period between November 2012 and October 2013. Ulcerative colitis patients were diagnosed on the basis of clinical, endoscopic and histological manifestations according to the criteria of American Gastroenterology Association [10]. They were divided according to endoscopic and histopathological findings into: Group I: 27 patients with mild to moderate ulcerative colitis disease. Group II: 23 patients with severe ulcerative colitis disease. Group III: 10 individuals whose colonoscopic and histopathologic findings were normal as control. The following categories were excluded from the study: Pregnancy, malignancy, heart failure, renal failure, thyroid disorders, acute infection and stroke and patients with immunosuppressive drugs.
All patients and control were subjected to complete history taking and thorough clinical examination. Laboratory investigations including, complete blood picture, blood urea and serum creatinine, erythrocyte sedimentation rate and stool examination to exclude bacterial causes of colitis. Colonoscopy was performed in all groups and the severity of the disease was determined. An endoscopic scoring system for UC of Pine ton de Chambrun et al [11] was used. Score O: Normal or inactive disease. Score 1: Mild disease (erythema, decreased vascular pattern and mild friability). Score 2: Moderate disease (marked erythema, increased vascular pattern, friability and erosion). Score 3: Severe disease (spontaneous bleeding and ulceration). Endoscopic findings were recorded and multiple biopsies were taken for histopathology, IL-23p19 immunohistochemical staining and IL-23 R expression by flow cytometry.
Histopathological study
4-μm-thick serial sections of formalin fixed, paraffin-embedded tissue were cut and stained by Hematoxylin and Eosin for histopathological evaluation and grading of the groups.
A six grade classification system for inflammation was used. The grades were: 0, structural change only; 1, chronic inflammation; 2, lamina propria neutrophils; 3, neutrophils in epithelium; 4, crypt destruction; and 5, erosions or ulcers [12].
IL-23p19 Immunohistochemical staining
4-μm-thick serial sections of formalin fixed, paraffin-embedded tissue were cut and mounted on positively charged glass slides. After incubation at 60°C overnight and deparaffinization, sections placed in 0.01 M sodium citrate buffer (pH 6.0) and heated twice for 5 minutes in a microwave oven. After inactivation of endogenous peroxidase with 0.5% metaperiodic acid in Phosphate-Buffered Saline (PBS) for 10 minutes, sections were incubated with 10% horse serum in PBS for 1 hour Sections were incubated at 4°C overnight with 100x diluted primary goat anti-mouse IL-23 p19 antibody (R&D Systems, Inc.). The standard Avidin-Biotinperoxidase Complex (ABC) technique was performed using the Lab Vision Secondary Detection Kit (Ultra Vision Detection System Anti-polyvalent, HRP). The color was visualized by incubation with chromogen 3, 3' diaminobenzidine for 5 minutes. The slides were then counterstained with Mayer hematoxylin and cover slipped with Per mount (Stat Lab, McKinney, TX). Negative controls were set for each test without the primary antibodies.
Immunohistochemical evaluation
Results were expressed semi-quantitatively. Positively stained cells were counted by examining at least 10 random fields (X200) in each section and expressed as the percentage of positive cells over total cell number [13].
IL-23 R expression by flow cytometry
Peripheral Blood Mononuclear Cells (PBMC)
CD4+ T isolation from blood samples: Lymphocytes were isolated from peripheral blood by incubation with Rosette Sep Human CD4+T cells enrichment cocktail (Stem Cells Technologies, Grenoble, France) followed by centrifugation on a density gradient (Lymphoprep, PAA, Pasching, Austria). Lymphocytes were purified by centrifugation through Lymphoprep.
For peripheral CD4+: For cellular surface staining the following antibodies and secondary reagents were used in different combinations: biotinylated goat anti-human IL-23R (BAF1400, R&D System, Minneapolis, MN). Streptavidin-APC (BD Bioscience, San Jose, CA), CD3-FITC (eBioscience, San Diego, CA), CD4 PE-Texas Red (Invitrogen, Carlsbad, CA), CD45RO-FITC (Dako, Glostrup, Denmark), CD45RO-Pacific Blue (BioLegend, San Diego, CA), CCR6-PE (BD Bioscience), CD45RA-PE (Invitrogen), CD45RAPE- Cy7 (eBioscience), plus matched isotypes as controls. Cells were acquired on a BD FACSAria II (BD Bioscience). Analysis of FACS data was performed by Flow Jo (Tree Star, Ashland, OR) software.
Lamina Propria Mononuclear Cells (LPMC)
Isolation of intestinal lymphocytes: For IEL isolation, endoscopic procedure was done, tissue (dissected mucosa) was placed and stirred for 15 minutes at room temperature in prewar med (37°C) 1x HBSS containing 10% fetal bovine serum (Gibco catalog no. 10082), 0.015 M HEPES, and 5 mM EDTA and stirred for 15 minutes at 37°C, followed by three 15-minutes washes with buffer adjusted to room temperature. The supernatant from each wash was pooled and poured through a nylon wool column to enrich for T cells and remove mucus. The resulting cell suspension was used to analyze IEL.
For IEL: The IEL suspensions containing approximately 4 x 106 cells each were re suspended in cold phosphate-buffered saline and stained with Aqua Live/Dead cell discriminator (Invitrogen catalog no. L34597) according to the manufacturer's protocol. Cells were then stained for 1 hour in the dark at 4°C with optimized concentrations of anti-CD3 Alexa750-APC (eBioscience clone 17A2), anti-CD8 Alexa700 (eBioscience clone 53-6.7), anti-CD4 Pacific Blue (eBioscience clone RM4-5), anti-TCR GD R-PE (BD Pharmingen clone GL3), and biotinylated polyclonal anti-IL-23R (BAF1686; R&D Systems). Cells were washed twice with PBS containing 1% bovine serum albumin (fluorescence-activated cell sorting [FACS] buffer). Cells were then stained for 1 hour with streptavidin-conjugated Q dot 605 (Q10101MP; Invitrogen). Stained cells were washed once in FACS buffer and subsequently fixed in 4% formalin for 1 hour. Cells were then washed once and re suspended in FACS buffer and analyzed using an LSR II flow cytometer (Becton Dickinson, San Jose, CA). The data were analyzed by using Flow Jo software (Tree star, Inc., Ashland, OR). Gates were set on singlet's and then on live lymphocytes. Subsequent gates were based on Fluorescence-Minus- One and unstained controls.
Serum IL-23 by ELISA
IL-23 cytokine level was assayed using a commercially available IL-22 ELISA kit (R&D Systems) according to manufacturers' instructions. All patients gave their informed consents and the study was approved by Ethical, and Research Committee, Tanta Faculty of Medicine, Tanta, Egypt.
Statistical analysis
The statistical data are reported as the mean ± SD, frequencies (number) and percentages when appropriate. A comparison of numerical variables between the study groups was performed using Mann-Whitney U test to compare independent samples from two groups. One-way analysis of variance test was used to compare between more than two groups when data were normal and the Kruskal-Wallis test when the data were not normal while qualitative data compared with chi square test. Spearman's rank correlation was used to quantify the association between continuous or ordered categorical variables. A P-value less than 0.05 were considered statistically significant. All statistical calculations were performed using the computer program SPSS (Statistical Package for the Social Science; SPSS, Chicago, IL, USA) version 15 for Microsoft Windows.
Results
Clinical and laboratory data of the studied groups (Table 1)
Significant higher levels of ESR and disease duration were detected in patients with severe disease compared to patients with mild to moderate ulcerative colitis disease P < 0. 05. Abdominal pain, diarrhea, blood in the stool and ESR were significantly higher in ulcerative colitis patients compared with control.
Parameters
Mild-moderate UC
Group I
(n =27)
Severe UC
Group II
(n =23)
Control Group
Group III
(n =10)
P-value
Age (ys)
Sex (m/f)
Disease duration(ys)
Abdominal pain
Diarrhea
Blood in stool
ESR (mm/hr)
Hb (gm/dl)
36.1 ± 13.4
16/11
2.6± 1.7
20/27
18/27
19/27
21.8± 7.9
12.1± 1.3
41.5± 12.7
14/9
4.2±3.1b
18/23
19/23
21/23
33.6± 8.5b
11.6± 1.6
35.2± 12.8
6/4
-
3a
2a
-
10.9± 1.9a
12.6±1.5
0.270
1.000
0.042b
0.083
0.017*
0.111
<0.001*
0.197
P < 0.05*significant between all groups; asig between patients & control;bsig between GI & GII.
UC: Ulcerative Colitis
Table 1: Clinical and laboratory data of the studied groups.
Histopathological evaluation and correlation with endoscopic groups (Table 2)
The studied 50 cases of UC showed different grades of severity 5 cases were grade 0 (10%); 12 cases were grade 1 (24%); 8 cases were grade 2 (16%), 15 cases were grade 3 (30%) (Figure 1a); 6 cases were grade 4 (12%) %) (Figure 1b) and only 4 cases were grade 5 (8%) %) (Figure 1c). In correlation with the endoscopic grouping; in group I; the cases distributed among grades 0-3; while in group II; the cases were distributed among grades 2-5 with statistical significance (P=0.02).
Studied groups
Histopathological grading
P -value
Grade 0
Grade 1
Grade 2
Grade 3
Grade 4
Grade 5
Mild-moderate UC
Group I
(n=27)
5
12
6
4
0
0
0.02*
Severe UC
Group II
(n=23)
0
0
2
11
6
4
P < 0.05 *significant; UC: Ulcerative Colitis
Table 2: Correlation between histological grading and endoscopic severity in the UC patients.
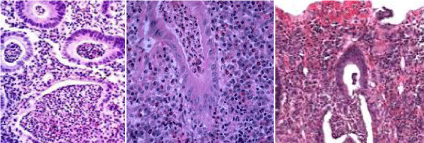
Figure 1: Histopathological grades of ulcerative colitis showing (a): grade
3 with neutrophilic infiltrate in the epithelial cells, (b): grade 4 showing crypt
destruction and (c): grade 5 showing erosions and ulcers. [H&E]
IL-23 R expression, serum IL-23 and IL-23p19 expression in the studied groups (Table 3)
(Table 3) shows significant increase in serum level of IL- 23and IL-23R expression in both peripheral blood and lamina propria lymphocyte (CD4) in ulcerative colitis patients compared with control group. This increase was more in patients with severe disease compared with patients with mild to moderate disease. IL- 23 p 19 Immunohistochemical evaluation showed that IL23p19 was expressed in the lamina propria macrophages. The mean number of the cells expressing IL23p19 in group I was 11.6±3.4 (Figure 2a) while in group II it was 19.6± 5.5 (Figure 2b) with statistical difference between the two groups. On the other hand, it was only 3.5± 1.6 in group III (control group) (Figure 2c) with statistical difference between the three groups.
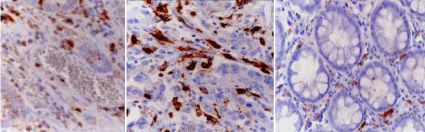
Figure 2: Immunohistochemical expression of IL23p19 in ulcerative colitis
groups showing mild expression in group I (a), extensive expression in group
II (b) and very minimal expression in group III (c). [IHC]
Parameters
Mild-moderate UC
Group I
(n =27)
Severe UC
Group II
( n =23)
Control Group
Group III
( n =10)
P-value
-IL-23 R expression %
.PBMC
.LPMC
-S IL-23 (pg/ml)
-IL-23 p 19 expression
Immunohistochemestry
% of +ve cells
6
20
48.9±7.6
11.6±3.4
12
29
137.4±45.5b
19.6± 5.5b
1c
6a
243.2±90.4a
3.5± 1.6a
0.037*
0.001*
<0.001*
<0.001*
P < 0.05*significant between all groups: a sig between patients & control; bsig between GI & GII. csig between GII & control; UC: Ulcerative Colitis; PBMC: Peripheral Blood Mononuclear Cells (lymphocyte); LPMC: Lamina Propria Mononuclear Cells (lymphocyte)
Table 3: IL-23 R expression, serum IL-23 and IL-23p19 expression in the studied groups
Correlation between endoscopic severity, histopathological grading and studied parameters in UC patients (Table 4)
(Table 4) shows significant positive correlation between both endoscopic severity and histological grading of ulcerative colitis with each of IL-23p19 expression ( r=0.591, p=0.038) and , (r=0.612, p=0.047) respectively, IL-23 R expression (r=0.542, p=0.015) and (r=0.526, p=0.031) respectively, serum level of IL-23 (r=0.637, p=0.024) and (r=0.551, p=0.019) respectively.
Parameters
IL-23p19 gene expression
IL-23 R expression
Serum IL-23
R
P-value
R
P-value
R
P-value
-Endoscopic severity
-Histopathological grade
0.591
0.612
0.038*
0.047*
0.542
0.526
0.015*
0.031*
0.637
0.551
0.024*
0.019*
P < 0.05 *significant; UC: Ulcerative Colitis.
Table 4: Correlation between endoscopic severity, histopathological grading and studied parameters in UC patients.
Discussion
Patients with UC are at increased risk of inflammation. IL- 23 is a newly identified cytokine with increased expression in inflamed biopsies of colon mucosa in patients with CD; however there is inconsistent evidence on its role in ulcerative colitis [14]. IL-23 is a heterodimeric cytokine that shows similar function to IL-12 in promoting cellular immunity and enhancing lymphocyte proliferation [15].
This study showed significant increased expression of IL-23p 19 gene in patients with UC compared with control. This increase was significantly higher in patients with severe UC disease compared with mild to moderate disease. This was similar to the results reported by Schmidt et al [16], Zhanju et al [13] and Kobayashi et al [17]. We observed that IL-23 p19 positive cells by immunohistochemistry were mainly macrophages. This was in agreement with Zhanju et al [13]. These findings suggest that IL-23 is produced by intestinal mucosal macrophages in inflamed mucosa of ulcerative colitis patients [13]. Upon stimulation by bacterial ligand, IL-23 is produced by antigen presenting cells. After binding to appropriate receptor (IL-23 R), this cytokine can stimulate the production of IL-17, TNF alpha and IL-6 from T cells. IL-17 stimulates the expression of adhesion molecules like ICAM-1 on endothelial cells, as well as the release of IL-6 and IL-8 from myofibroblast and epithelial cells. IL-8 act as chemo tactic factor for neutrophil in flux in to the intestine. Inflammatory neutrophil release inflammatory mediators like matrix metalloproteinase and inducible nitric oxide. This sequel of pathogenic events leads to the chronic inflammation and epithelial cell damage associated with the disease [18]. Therefore, IL-23 was proposed to play an integral role in the pathogenesis of IBD [19].
This study showed the increased expression of IL-23 R in both peripheral blood and mucosal biopsy of ulcerative colitis patients were significant compared with control group. This increased expression was higher in patients with severe disease compared with those have mild to moderate disease. Also, Zhanju et al [13] demonstrated significant increased expression of IL-23 R in peripheral blood and mucosal lamina propria cells.
In accordance with the findings reported by Mohammadi et al [14] and Zheng et al [20], we demonstrated increased serum level of IL-23 in ulcerative colitis patients compared with control. In addition, the increased serum levels of IL-23 were more in patients with severe disease compared with mild to moderate disease. Moreover, this study reported significant positive correlation between high serum IL-23 levels and endoscopic severity of the disease. These findings support the hypothesis that, increased IL-23 levels reflect the activity of T helper 17 in patients with ulcerative colitis and that IL-23 participate in the pathogenesis of the disease [20]. This study noticed, the increased expression of IL-23p19 and IL-23 R were significantly positively correlated with endoscopic severity of the disease. These were consistent with Schmidt et al [16] and Zhanju et al [13]. In consistent with experimental studies of Daniel et al [21] and Ando et al [22], there were significant positive correlation between histopathological severity and both IL-23p19, IL-23R expression in the mucosa of ulcerative colitis patients. Furthermore the severity of histopathological lesions was significantly correlated with serum IL- 23, similar to an experimental study of Sheikh et al [23].
It can be concluded that the increased expression of IL-23p19 has a role in the pathogenesis of ulcerative colitis; therefore targeted therapy directed against IL-23p19 may have a therapeutic role for the disease. The increased expression of IL-23p19, IL-23 R and serum IL- 23 correlate with disease severity.
References
- Shih DQ, Targan SR. Insights into IBD Pathogenesis. Curr Gastroenterol Rep. 2009; 11: 473-480.
- Nagahori M, Nemoto Y, Watanabe M. Pathogenesis of inflammatory bowel disease. Intest Res. 2010; 8: 9-17.
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000; 13: 715-725.
- Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002; 168: 5699-5708.
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T (H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007; 445: 648-651.
- Langrish CL, Chen Y, Blumenschein WM, Jeanine Mattson, Beth Basham, Jonathan D, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201: 233-240.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009; 27: 485-517.
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008; 118: 3537-3545.
- Hue S, Ahern P, Buonocore S, Marika C. Kullberg, Daniel J. Cua, Brent S. McKenzie, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006; 203: 2473-2483.
- Kornbluth A, Sacher DB. Ulcerative colitis practice guidelines in adult: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105: 501-523.
- Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010; 7: 15-29.
- Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000; 47: 404-409.
- Zhanju Liu, Yadav P, XiaorongXu, Jingling Su, Chi Chen, Maochun Tang, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leuk Biol. 2011; 89: 597-606.
- Mohammadi M, Hayatbakhsh MM, Zahedi MJ, Jalalpour MR, Pakgohar A. Serum interleukin-23 levels in patients with ulcerative colitis. Iran J Immunol. 2011; 8: 183-188.
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003; 278: 1910-1914.
- Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005; 11:16-23.
- Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008; 57: 1682-1689.
- Fitzpatrick LR. Novel Pharmacological Approaches for Inflammatory Bowel Disease: Targeting Key Intracellular Pathways and the IL-23/IL-17 Axis. Int J Inflam. 2012; 389404.
- McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007; 56: 1333-1336.
- Zheng ZD, Wan XQ, Liu LY. Serum contents of IL-23 and IL-17 in the patients with ulcerative colitis and the clinical significance. 2011; 27: 203-206.
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008; 324: 23-33.
- Ando Y, Yang GX, Tsuda M, Kawata K, Zhang W, Nakajima T, et al. The immunobiology of colitis and cholangitis in interleukin-23p19 and interleukin-17A deleted dominant negative form of transforming growth factor beta receptor type II mice. Hepatology. 2012; 56: 1418-1426.
- Sheikh SZ, Matsuoka K, Kobayashi T, Li F, Rubinas T, Plevy SE, et al. Cutting edge: IFN-gamma is a negative regulator of IL-23 in murine macrophages and experimental colitis. J Immunol. 2010; 184: 4069-4073.