
Research Article
Austin J Drug Abuse and Addict. 2016; 3(1): 1009.
Self-reported Sleep Improvement in Buprenorphine MAT (Medication Assisted Treatment) Population
Zheng WH¹*, Wakim RJ¹, Geary RC¹, Lander LR¹, Wen SJ², Xiao MC² and Sullivan CR¹
¹Department of Behavioral Medicine and Psychiatry, West Virginia University, USA
²Department of Biostatistics, West Virginia University, USA
*Corresponding author: Zheng WH, Department of Behavioral Medicine and Psychiatry, West Virginia University, 930 Chestnut Ridge Road, USA
Received: May 26, 2016; Accepted: July 18, 2016; Published: July 25, 2016
Abstract
This is a prospective, naturalistic study to evaluate patient’s report on sleep and depression in early recovery while receiving buprenorphine in Medication Assisted Treatment (MAT). 40 Subjects entering into MAT with buprenorphine/ naloxonefor opioid dependence disorder were recruited. No change of concurrent treatment was made. Subjects were administered Sleep Scale from the Medical Outcomes Study (MOS-Sleep), a 5-item Supplemental Sleep Scale (SSS), and the Beck Depression Inventory II (BDI-II). The measures were administered at day 0 (baseline), 30, 60 and 90 days.The result showed that patients reported significant progressive improvements in three MOSSleep subscales: sleep disturbance, sleep indices I and II. The mean scores of SLPD4 (Sleep disturbance) at day 0, 30, 60, 90 were 62.4, 53.2, 53.3, and 48.4 respectively (p=0.0029). Similarly, subscores of SLP6 (Sleep Problem Index I) and SLP 9 (Sleep Problem Index II) were also significantly decreased over time (P=0.038 for SLP6 and p=0.007 for SLP9). BDI-II depression scores improved from “Moderate depression” at baseline to “Mild depression”. The mean BDI score decreased from 24.2 to 17.0 after 90 days of treatment. Findings suggest that subjects reported improvement in both sleep and depression after initiating MAT with buprenorphine/naloxone.
Keywords: Buprenorphine; MAT; Sleep; Depression; MOS-Sleep; BDI-II
Abbreviations
MAT: Medication Assisted Treatment; MMT: Methadone Maintenance Therapy; PSQI: Pittsburgh Sleep Quality Index; COAT: Comprehensive Opioid Addiction Treatment; TRD: Treatment Resistant Depression; MOS-Sleep: Sleep Scale from the Medical Outcomes Study; SSS: Supplemental Sleep Scale; BDI-II: Beck’s Depression Inventory II
Introduction
Opioid dependence disorders have become an epidemic public health concern in the United States. In 2014, 4.3 million Americans age 12 and up, representing 1.6% of the population, reported current non-medical use of prescription pain relievers, while 435,000 people had heroin addiction [1]. Patients with opioid use disorder experience significant sleep distress and depressive mood in various stages: intoxication, withdrawal, chronic use, early recovery and even maintenance therapy [2-6]. This can often be a trigger for relapse and affect the success of treatment. The psycho- and patho- physiologies of sleep impairment in individuals with opioid dependence disorder are multifaceted and complicated. It is always a clinical challenge to determine cause and effect among sleep, depression, and substance use. Chronic substance use disrupts circadian rhythms and thereby impairs the normal sleep-wake cycle [7]. Reports on the direct effect of opioids on sleep quality and efficiency are inconsistent and therefore inconclusive. There has been evidence to support opioid use as a cause of sleep disordered breathing [8], and as a contributor to significant reduction in REM and Stage 3 slow wave sleep as well as to increased risk of central sleep apnea [9,10]. Opioid use has also been associated with increased nocturnal wake times [11]. These effects could be dose related because association was found between prescription opioid status and dose and self-reported sleep impairment [12].
Methadone is a synthetic full opioid agonist that can mitigate opioid withdrawal symptoms. It has been used successfully for more than 40 years in the treatment of opioid dependence [13,14]. Poor sleep function has been reported by patients receiving Methadone Maintenance Therapy (MMT) [15,16]. One study identified that the frequent complaint of sleep disturbance in this population was supported by objective sleep measures such as home polysomnography thereby showing that this population is reliable in evaluating their sleep disturbance [17]. Another study analyzed the correlation between related factors such as duration of opiate exposure, length of time in MMT, current methadone dosage and the severity of sleep disorders. The results showed that more than 70% of patients were poor sleepers and the Pittsburgh Sleep Quality Index (PSQI) and scores correlated significantly with the methadone dosage [18].
Buprenorphine, a partial μ-opioid agonist with limited respiratory toxicity, has been widely used in effective Medication Assisted Treatment (MAT) for opioid dependence disorder. It is associated with many favorable outcomes [19-24]. Like that of methadone, the effect of buprenorphine on sleep disturbance remains unclear. One cross-sectional observational study suggested that buprenorphine/ naloxone might induce significant CSA (Central Sleep Apnea) and hypoxemia [25]. Conversely, one case report indicated successful reversal of CSA with change from methadone to buprenorphinenaloxone [26]. An early study of buprenorphine treatment in heroin dependent men suggested that it could improve sleep disturbance and normalize sleep patterns [27]. Another relevant study showed that slower taper during buprenorphine detoxification correlated with significantly fewer disruptions in sleep compared to the faster tapering experimental groups [28]. Whether buprenorphine has direct effect on breathing and may cause worsening sleep [29] or if it can improve sleep quality by a complicated and unknown mechanism [26-30] is uncertain. Regardless, with increasing use and popularity of buprenorphine in MAT, sleep disturbance and its effect on opioid dependence and early recovery present a unique challenge to the treatment of this population and warrant further research studies.
The relationship between opioid addiction and depression could be very complicated. On one hand, opioid dependence patients often have comorbid depression that may be secondary or primary in the etiology of their addiction problem [31,32]. On the other hand, depression could be a common psychiatric complication in addiction, and one of serious symptoms of opioid use and withdrawal, constituting a firm reinforcement of continued addiction [33-35]. Moreover, the loss of self-control, shame and guilty, helplessness, hopelessness, subsequent disruption of job performance and social ties, even criminal behavior can all cause addicted people to suffer from worsening mood deterioration and dysphoria. Interestingly, buprenorphine as a potent kappa antagonist has been shown to have antidepressant activity [36,37]. A recent open label clinical trial demonstrated that buprenorphine combined with a mu opioid antagonist significantly reduced depressive symptoms in individuals with Treatment Resistant Depression (TRD) [38]. This indicates that modulation of the opiate system has something to do with depression and using buprenorphine like product may be a novel treatment approach for TRD. Sleep problem, as one of the diagnostic symptoms of depression, is also a risk factor, consequence, and complication of both depression and drug addiction [39,40]. Often it serves as a prognostic indicator of long-term depression course and treatment response. Although insomnia usually improves as depression is treated, it may persist in addiction patients, indicating a separate etiology and need for further intervention. The above all add complexity to the study of sleep in MAT population but also suggest greater need for thorough mood assessment at MAT treatment entry and continuous close monitoring throughout the treatment course.
The primary outcome investigated in the present study was the effect of buprenorphine used in MAT on sleep. We hypothesized that patients would report poor sleep at entry into treatment and show improvement in sleep over time. The secondary outcome measured was the effect of buprenorphine used in MAT in depression. We hypothesized subjects would report improvement in depression as assessed by the Beck Depression Inventory II (BDI-II).
Method
Study setting and participants
This study was completed at West Virginia University Department of Behavioral Medicine and Psychiatry Chestnut Ridge Center, one of the largest mental health service centers in West Virginia. The center provides outpatient opioid addiction treatment through interdisciplinary team approach named the Comprehensive Opioid Addiction Treatment (COAT) program. It includes group based medication management followed by substance abuse focused group therapy. Patients are also required to participate in 12-step peer support meetings. All participants were diagnosed with Opioid Dependence (DSM IV) and at the time of the study were entering into COAT MAT using buprenorphine/naloxone. Per prescribing information [41], the inclusion of naloxone in the sublingual formulation is to help deter the possible diversion of buprenorphine to misuse by the parenteral route. Although blood concentration might be measureable, when administered by the sublingual route, naloxone was reported to have no clinically significant effect. Therefore we only refer to the active ingredient buprenorphine for this research purpose. Informed consent was obtained prior to participation. Subjects received no monetary compensation. The only exclusion criterion was active pregnancy. No change in concurrent treatment was made in hopes that this would allow for a more representative sample of outpatients in “real world” practice. Per clinic policy, we maintain a list of medications (including most of the controlled substances such as Benzodiazepines, Z-drugs, stimulants, etc.) that were disallowed for the purpose of buprenorphine maintenance treatment. To ensure compliance and avoid drug diversion, patients are subject to random pill counts and random urine drug screens. Buprenorphine and its metabolite nor-buprenorphine must be present in the urine screen in order for new prescription to be written. The study was consistent with protection of patient rights per Health Insurance Portability and Accountability Act and was approved by the West Virginia University institutional review boards.
Materials
Sleep scale from the Medical Outcomes Study (MOSSleep)
The MOS-Sleep is a 12-item self-report questionnaire that measures six dimensions of sleep: “sleep disturbance,” “snoring,” “sleep awakening short of breath or with headache,” “sleep adequacy,” “somnolence,” and “quantity of sleep/optimal sleep” [42,43]. Based on a comprehensive conceptual model that covers both physical and mental health, it was originally designed for patients with chronic illness but was also validated in general population as an effective approach to the sleep assessment [42,44-47]. The item and subscale contents of the MOS-Sleep are presented in (Table 1) [43].
Item
Item contents
1
Time to fall asleep
2
Quantity of sleep
3
Sleep restlessness
4
Enough sleep, feel rested
5
Awaken shortness of breath or headache
6
Feel drowsy
7
Trouble falling asleep
8
Awaken during sleep
9
Trouble staying awake
10
Snore during sleep
11
Take naps
12
Amount sleep needed
Subscale
Item(s)
SLPD4
Sleep disturbance
1, 3(R), 7(R), 8(R)
SLPSNR1
Snoring
10(R)
SLPSOB1
Sleep shortness of breath or headache
5(R)
SLPA2
Sleep adequacy
4(R), 12(R)
SLPS3
Sleep somnolence
6(R), 9(R), 11(R)
SLP6
Sleep problem index I
4, 5(R), 7(R), 8(R), 9(R), 12
SLP9
Sleep problem index II
1, 3(R), 4, 5(R), 6(R), 7(R), 8(R), 9(R), 12
(R) Refers to a reversed item.
Table 1: MOS-Sleep item and subscale content.
Scoring the MOS-Sleep is a two-step process. The first step is to recalibrate the recorded numeric values from the survey into a 0-100 scale, which represents the achieved percentage of the total possible score. Secondly, items within each scale are averaged together, some were reversely calculated, to create seven subscale scores: SLPD4 (Sleep Disturbance), SLPNR1 (Snoring), SLPSOB1 (Sleep Shortness of Breath or Headache), SLPA2 (Sleep Adequacy), SLPS3 (Sleep Somnolence), SLP6 (Sleep Problem Index I) and SLP9 (Sleep Problem Index II). The number in each subscale name indicates how many question items it contains. For all subscales except SLPA2, higher scores reflect more abnormal. Item 2 is scored separately and used as is because it is a direct numeric answer from subject measuring average hours of sleep each night during the past four weeks. SLPD4 combined 4 items to reflect sleep disturbance; same for SLPSNR1, SLPSOB1 and SLPS3 higher scores reflect more of the attribute implied by the names: greater snoring during sleep in SLPSNR1, worse awaken shortness of breath or headache in SLPSOB1 and more somnolence (trouble staying awake and feeling drowsy) in SLPS3. 6-item Sleep Problem Index I (SLP6) and comprehensive 9-item Sleep Problem Index II (SLP9) summarize information from domains of sleep disturbance, sleep adequacy, shortness of breath and somnolence into a single score. Both indices cover same domains but differ in length. Index-I eliminates potentially overlapping items but still proves to be reliable.
Supplemental Sleep Scale (SSS)
A five-item supplemental sleep scale was designed to inquire information about patient’s own perception of overall sleep quality, sleep improvement, relationship between sleep change and buprenorphine use. Substance use in the past 30 days was also assessed to capture relapse during recovery process. This was also partially verified by the random urine drug screen results. Our experience showed that we obtained more relapse reports than positive urine drug screen results due to patient’s fear of being discharged from the COAT program.
Beck’s Depression Inventory II (BDI-II)
The Beck Depression Inventory II (BDI-II) is a commonly used self-report instrument for depression evaluation. It is highly reliable and has been tested for content, concurrent and constructs validity [48]. It includes 21 items answered in a four-point scale. It assesses both psychological and physical symptoms. Total score can be matched to four levels of depression ranging from “minimal depression” to “severe depression”. In this study, BDI-II was used to measure depression during the buprenorphine MAT treatment.
Procedure
This is a prospective, naturalistic study. No specific interventions or changes were made except the administering of surveys. Eligible subjects were given the aforementioned three instruments and asked to complete the questionnaires in paper format at four time points: at intake assessment for MAT (baseline), then 30, 60, and 90 days into treatment. Per policy of the COAT program treatment, they were all in initial recovery group and were seen by psychiatrist weekly and were required to participate in a group therapy and four 12-step peer support meetings per week. All participants were also subject to random urine drug screens and pill counts. At any time, patients may choose to drop out of the program voluntarily or be discharged at the treatment team’s discretion, based on treatment compliance and dishonesty about drug and alcohol use.
Data analysis
The primary endpoint is the sleeping scores and the primary analysis is to assess the score change over time. Depression was assessed as the secondary outcome. Descriptive statistics were used to describe sleep and depression improvement in all self-report scales, including mean with standard deviation and range for continuous variables such as MOS scores and BDI-II total scores, and proportions or percentage for categorical variables. In univariate data analysis, a paired T test was used to examine the differences of scores between day 0 (baseline) and day 30, 60, 90, respectively. A bar-plot was used to explore the distribution of scores at day 30, 60, 90 in comparison with baseline. In the multivariate data analysis, a mixed-effects model accounting for subject effects was used to analyze the longitudinal data on sleeping scores over time, adjusting for gender, age and dose, while a logistic model with Generalized Estimating Equation (GEE) method was used in additional substance use (Yes/No) analysis. A p-value < 0.05 implies the statistical significance in this study.
Statistical calculations were performed using SAS 9.2 and R software, version R 3.1.3.
Results
Table 2 presents the basic demographic information of subjects. Total forty patients (22 males and 18 females) consented and they were between ages of 19 and 62 years (M=35.3, SD =11.6). All subjects were maintained on buprenorphine/naloxone 8-16 mg per day.
Characteristic (N=40)
Mean±SD, or n (%)
Age
35.3±11.6
Gender
Female
18 (45%)
Male
22 (55%)
Race
White
39 (97.5%)
Non-white
1 (2.5%)
Employment Status
Employed
30 (75%)
Unemployed
10 (25%)
Education (N=38)
Less than high school or GED
8 (21.1%)
High school diploma or GED
20 (52.6%)
Some college or more
10 (26.3%)
Buprenorphine/naloxone Dose
11.9±2.3
8mg
7 (17.5%)
10mg
1 (2.5%)
12mg
14mg
16mg
23 (57.5%)
5(12.5%)
4 (10%)
Table 2: Population basic characteristics.
MOS-Sleep subscales
Figure 1 depicts the average MOS-Sleep subscale score changes at four different study points. SLPD4, SLP6, and SLP9 demonstrate clear downslope changes indicative of sleep improvement over time with buprenorphine treatment. The mean scores of SLPD4 (Sleep Disturbance) at day 0, 30, 60 and 90 were 62.4, 53.2, 53.3 and 48.4 respectively (p=0.0029). SLPA2 reflects sleep adequacy and is reversely calculated from two items. The increasing score shown in the graph was consistent with less sleep problem over time in early buprenorphine treatment but was not statistically significant. Subscales SLPSNR1, SLPSOB1, and SLPS3 have fluctuations and changes were tested as non-significant as well.
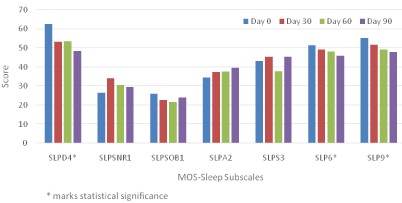
Figure 1: MOS-Sleep subscale score change.
A fitted mixed-effects model of SLPD4, SLP6, and SLP9 over time, adjusting for gender, age and dose was used to study the changes in these three subscales. The effects on time were found to be all negative, which implies that all three sleeping scores were significantly decreasing over time with the effects equal to -5.20 in SLPD4 (p = 0.0029), -3.15 in SLP6 (p = 0.038) and -3.69 in SLP9 (p = 0.0070) respectively. The fitted regression lines for the three sleeping scores over time are shown in (Figure 2), based on the results from the mixed models.
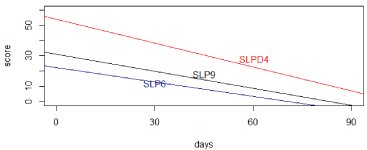
Figure 2: Fitted regression lines for three sleep subscores over time.
Beck Depression Inventory II (BDI-II)
(Table 3) shows a longitudinal follow up of BDI-II. Patients reported “Moderate depression” (BDI score range 20-28) at baseline. This decreased to “Mild depression” (BDI-II score range 14-19) after 30 days treatment, and continued to stay at same level over 90 days. The percentage change was 31-35%. Moreover, a mixed-effects model adjusting for gender, age and dose supported this conclusion (p=0.0001).
Time (day)
Mean (range)
P*
0
24.2 (6, 47)
-
30
17.2 (0, 39)
0.0001
60
17.5 (1, 40)
0.0012
90
17.0 (1, 44)
0.0021
*p: p-value for comparison with baseline (Day 0)
Table 3: Beck Depression Inventory II (BDI-II) scores follow up.
Supplemental Sleep Scale (SSS): additional substance use, perceived relationship between buprenorphine/ naloxone use and sleep change
One question of the Supplemental Sleep Scale (SSS) asked about last month use of any additional substance not allowed during buprenorphine MAT. We dichotomized it as a binomial variable (Yes/No). A logistic model with the Generalized Estimating Equation (GEE) method was used in the longitudinal data analysis. The result shows that the percentage of self-reported additional substance use was significantly reduced over time (p = 0.034). The counts and percentages of self-reported additional substance use (at least once) in the past 30 days at each follow up time points were summarized in first section of (Table 4).
Time (day)
Additional substance use
Perceived relationship between buprenorphineand sleep change
N
Count (%)
N
Count (%)
No
Yes
(1 – 2)*
(3 – 6)*
30
27
12 (44.4%)
15 (55.6%)
29
16 (55.2%)
13 (44.8%)
60
21
11 (52.3%)
10 (47.6%)
22
14 (63.6%)
8 (36.4%)
90
20
12 (60%)
8 (40%)
20
10 (50%)
10 (50%)
*Scores: 1=not at all; 2=a little related; 3=some related; 4=a good bit related; 5=mostly related; 6=completely related.
Table 4: Supplemental Sleep Scale (SSS) result summary.
Another question in the same scale inquired about whether subject believed that his/her sleep during the last month was related to the use buprenorphine/naloxone. It was rated in a 6-point scale from “not at all related” (0 point) to “completely related” (6 points). The second section of (Table 4) shows that at day 90, 50% participants reported that their sleep change was “some” to “completely” relate to use of buprenorphine/naloxone.
Discussion
There has been inconsistency in the literature about buprenorphine and sleep change. This prospective study adds to the knowledge about using buprenorphine in real-world opioid dependence treatment by demonstrating that most patients reported improved sleep and many patients reported a perceived relationship between the changes in their sleep and being on buprenorphine (36.5% to 50%), the strongest perceived relationship being at day 90.The greatest statistically significant improvement in sleep was in the first 30 days of treatment and the trend continued through 90 days. Sleep disturbance decreased; snoring, shortness of breath and headache showed no significant statistical difference; sleep adequacy in quality and amount increased; while reports of drowsiness remained variable. Fitted mixed-effects models showed overall sleep improvement adjusting for gender, age of medication dose. Depression also significantly decreased, again most drastically in the first 30 days of treatment and then plateaued by day 30 with the average BDI-II score remaining in the mild depression clinical range. Interestingly, at day 90 of treatment 50% of subjects believed their use of buprenorphine/naloxone was somewhat, a good bit or mostly responsible for their improved sleep which was an increase from those who had that perception at day 30 and 60.
While these results are promising, it is difficult to sort out what role buprenorphine itself plays in the improvement of sleep versus discontinued use of substances of abuse and the corresponding elimination of the withdrawal-intoxication cycle. Also, in active addiction individuals are known to have poor sleep hygiene, which likely contributes to their poor sleep scores. In addition, depression is highly correlated with sleep problems. 70 to 90% of people seeking treatment for Major Depressive Disorder suffer from insomnia and experience difficulties falling and staying asleep, or poor general sleep quality [39,49]. Upon entering treatment, subjects reported an average level of depression in the moderate range. By 90 days in treatment the majority of subjects who stayed in treatment reported depression in the minimal depression range. The significant improvement in depression among those subjects may also be a contributing factor to overall improved sleep.
Buprenorphine has a mean elimination half-life ranging from 31 to 35 hours. Although the medicine is usually recommended to be taken once a day, like many other opioid dependent patients, in our COAT clinic, each patient has a different dosing schedule. Records showed that while most of the patients take it once a day early in the morning or before bedtime, some split into 2-3 doses throughout the day. The clinicians were trained to educate and encourage patients to follow the same manner of dosing with continued use of the product to ensure consistency in bioavailability. While buprenorphine can cause drowsiness especially at the beginning of maintenance therapy, which we recognize as one of the potential confounding factors affecting our outcome analysis, usually the sedation side effect wears off quickly with treatment. Also our observations showed most of participants have already built up tolerance through past use of opioid products and rarely report this kind of adverse reaction.
As mentioned in the introduction, buprenorphine/naloxone treatment has been reported to be associated with both increased risk [25] and reversal of central sleep apnea [26]. Our study did not have enough information to corroborate either of these but the result showed patients subjectively reported increased snoring in 30 days then this symptom tended to alleviate a bit and stayed the same at 60 and 90 day time points (Figure 1). For shortness of breath, during first 60 days into treatment, patients reported mild improvement but this did not last at 90 days. We cannot offer a complete explanation for these findings due to the scope of this study.
Data reported on additional substance use in (Table 4), indicates subjects were more likely to report relapse in the first 30 days of treatment (55.6%) and less likely by day 60 (47.6%) and even less so by 90 days of treatment (40%). This is inversely correlated with the greatest improvement of sleep and depression occurring in the first 30 days. This might suggest that the medication, not simply being clean and sober has an impact on sleep and depression. That being said, it is very difficult to tease out the impact that the group therapy may or may not have had on these outcomes.
The primary limitations of this study are the relatively small sample size and having no comparison group who were not receiving buprenorphine. We were not able to have a comparison group in this setting because patients all came seeking MAT and our setting is not licensed to prescribe methadone. The small sample size was in part due to the 50% dropout/discharge rate over the course of the 90 days. Primary reasons for drop-out/discharge were no shows orbeing untruthful about drug or alcohol use resulting in discharge. This dropout/discharge rate is consistent with other studies done in this population with the kind of rigorous requirements the COAT program has.
The results from the retained subjects indicate that use of buprenorphine/naloxone in MAT correlates with improved sleep and depression in the first 90 days of treatment, eventhough other factors such as therapy and/or peer support meetings may have also contributed. Given that this was an unfunded pilot study, we did not employ any objective sleep measures such as polysomnography which would help to avoid bias of self-report on sleep measures. In addition, there may have been unavoidable confounders such as overthe- counter or non-detectable prescription sleep aids or psychotropic medications such as antidepressants on which we did not collect data, with the original purpose of the study to mimic the real life situation. Future work can use a larger sample, randomized control groups, incorporate ways to identify factors potentially affecting sleep improvement and screen for use of adjunctive sleep aids. In addition, the use of both subjective and objective sleep measurements would be beneficial.
Conclusion
This study found that buprenorphine/naloxone maintained patients with opioid dependencedisorder in early recovery reported improvement in sleep and depression during the first 90 days treatment. A small preliminary naturalistic study of this kind is insufficient to draw any distinct conclusions regarding the direct effect of buprenorphine/naloxone on sleep and mood in this population; however, it adds to the literature given there has been so little research done in this area. Regardless of what the underlying causes are, this study adds knowledge to the previous findings of sleep disturbance during initial phase of treatment and confirms improvement in sleep quantity and quality with MAT in this population. It also has improved our awareness of some variables associated with recovery from opioid dependence disorder.
Acknowledgement
The authors thank Khalid Sharif, Arielle Stafford, Anthony Prasetio and Ashray Maniar for helping collect data. Also, this study was guided and assisted by Dr. James Berry during the initial IRB application phase. Data analysis was conducted by Dr. Sijin Wen whose work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- SAMHSA: Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. SAMHSA, USA. 2015.
- Puigdollers E, Salvany DA, Brugal MT, Torrens M, Alvaro’s J, Castillo C, et al. Characteristics of Heroin Addicts Entering Metha done Maintenance Treatment: Quality of Life and Gender. Substance use and misuse. 2004; 39: 1353-1368.
- Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in Methadone Maintenance Treatment (MMT) patients. Drug Alcohol Depend. 2006; 82: 103-110.
- Hartwell EE, Pfeifer JG, McCauley JL, Santa Maria MM, Back S. Sleep disturbances and pain among individuals with prescription opioid dependence. Addictive Behaviors. 2014; 39: 1537-1542.
- Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review. American Journal on Addictions. 2015; 24: 590-598.
- Tang JS, Liao YH, He HY, Deng QJ, Zhang GB, Qi C, et al. Sleeping problems in Chinese illicit drug dependent subjects. BMC Psychiatry. 2015; 15: 28.
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian Rhythms, Sleep and Substance Abuse. Sleep Medicine Reviews. 2012; 16: 67-81.
- Cheatle MD, Webster LR. Opioid Therapy and Sleep Disorders: Risks and Mitigation Strategies. Pain Medicine. 2015; 16: S22-S26.
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing (Clinical Review). Sleep Medicine Reviews. 2007; 11: 35–46.
- Dimsdale JE, Norman D, DeJardin D, Wallace MS. The Effects of Opioids on Sleep Architecture. Journal of Clinical Sleep Medicine. 2007; 3: 33-36.
- Moore P, Dimsdale JE. Opioids, sleep, and cancer-related fatigue. Medical Hypotheses. 2002; 58: 77-82.
- Morasco B, O'Hearn D, Turk D, Dobscha S. Associations between Prescription Opioid Use and Sleep Impairment among Veterans with Chronic Pain. Pain Medicine. 2014; 15: 1902-1910.
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999; 281: 1000–1005.
- Fullerton CA, Kim M, Thomas CP, Lyman R, Montejano LB, Dougherty RH, et al. Delphin-Rittmon. Medication-Assisted Treatment with Methadone: Assessing the Evidence. Psychiatric Services. 2014; 65: 146-157.
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, et al. Sleep disturbances among methadone maintained patients. Journal of Substance Abuse Treatment. 2004; 26: 175-180.
- BURKE CK, PEIRCE JM, KIDORF MS, NEUBAUER D, PUNJABI NM, STOLLER KB, ET AL. SLEEP PROBLEMS REPORTED BY PATIENTS ENTERING OPIOID AGONIST TREATMENT. JOURNAL OF SUBSTANCE ABUSE TREATMENT. 2008; 35: 328-333.
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug and Alcohol Dependence. 2011; 113: 245-248.
- Hsu WY, Chiu NY, Liu JT, Wang CH, Chang TG, Liao YC, et al. Sleep quality in heroin addicts under Methadone Maintenance Treatment. Acta Neuropsychiatrica. 2012; 24: 356-360.
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992; 267: 2750–2755.
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine and methadone for opioid dependence. N Engl J Med. 2000; 343: 1290-1297.
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. One-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003; 361: 662-668.
- Magura S, Lee SJ, Salsitz EA, Kolodny A, Whitley SD, Taubes T, et al. Outcomes of buprenorphine maintenance in office-based practice. J Addict Dis. 2007; 26: 13-23.
- Otiashvilia D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior-Outcomes of a randomized trial. Drug and Alcohol Dependence. 2013; 133: 376-382.
- Connery HS. Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry. 2015; 23: 63-75.
- Farney RJ, McDonald AM, Boyle KM, Snow GL, Nuttall RT, Coudreaut MF, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J. 2013; 42: 394-403.
- Wang D, Lintzeris N, Leung S, Haber PS, Yee BJ, Grunstein RR. Reversal of central sleep apnoea with change from methadone to buprenorphine-naloxone: a case report. Eur Respir J. 2015; 46: 1202-1205.
- Lukas SE, Dorsey CM, Mello NK, Mendelson JH, Lundahl LH, Sholar M, et al. Reversal of Sleep Disturbances in Cocaine and Heroin Dependent Men During Chronic Buprenorphine Treatment. Experimental and Clinical Psychopharmacology. 1996; 4: 413-420.
- Dunn K, Saulsgiver K, Miller M, NuzzoP, Sigmon S. Characterizing opioid withdrawal during double-blind buprenorphine detoxification. Drug and Alcohol Dependence. 2015; 151: 47-55.
- DeVido J, Connery H, Hill K. Sleep-disordered breathing in patients with opioid use disorders in long-term maintenance on buprenorphine-naloxone: A case series. Journal of Opioid Management. 2015; 11: 363-366.
- Yarlas A, Miller K, Wen W, Lynch SY, Ripa SR, Pergolizzi JV, et al. Buprenorphine Transdermal System Improves Sleep Quality and Reduces Sleep Disturbance in Patients with Moderate-to-Severe Chronic Low Back Pain: Results from Two Randomized Controlled Trials. Pain Practice Published online. 2015.
- Quello SB, Brady KT, Sonne SC. Mood Disorders and Substance Use Disorder: A Complex Comorbidity. Sci Pract Perspect. 2005; 3: 13-21.
- Nunes EV, Weiss RD. Co-occurring Addiction and Affective disorders. In: ?Principles of Addiction Medicine????. 2009; 1151-1182.?
- Goldsmith RJ, Ries RK, Flores YC. Substance-induced mental disorders. In: ?Principles of Addiction Medicine????. 2009; 1139-1150.?
- Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Kanof PD. The dysphoria of heroin addiction. Am J Drug Alcohol Abuse. 1992; 18: 275-287.
- Chavkin C, Koob GF. Dynorphin, Dysphoria and Dependence: the Stress of Addiction. Neuropsychopharmacology. 2016; 41: 373-374.
- Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, et al. Safety, tolerability and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014; 75: 785-793.
- Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. Journal of Substance Abuse Treatment. 1990; 7: 51-54.
- Ehrich E, Turncliff R, Du Y, Pemberton LR, Fernandez E, Jones R, et al. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology. 2015; 40: 1448-1455.
- Tsuno N, Besset A, Ritchie K. Sleep and Depression. Journal of Clinical Psychiatry. 2005; 66: 1254-1269.
- Riemann D, Berger M, Voderholzer U. Sleep and depression-results from psychobiological studies: an overview. Biological Psychology. 2001; 57: 67-103.
- Suboxone [packet insert]. Richmond, VA: Indivior Inc. 2015.
- Hays RD, Stewart AL. Sleep Measures: Measuring functioning and well-being; the medical outcomes study approach/edited by Stewart AL, Ware JE; Duke Edition. Duke University Press. 1992; 235-259.
- Spritzer KL, Hays RD. MOS Sleep Scale: A Manual for Use and Scoring, Version 1.0. RAND Corporation. 2003.
- Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Medicine. 2005; 6: 41-44.
- Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007; 36: 339-356.
- Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain. 2008; 137: 623-630.
- Sancisi E, Cevoli S, Vignatelli L, Nicodemo M, Pierangeli G, Zanigni S, et al. Increased prevalence of sleep disorders in chronic headache: a case-control study. Headache. 2010; 50: 1464-1472.
- Beck AT, Brown G, Steer RA. Beck Depression Inventory II manual. 1996.
- Thase ME, Murck H, Post A. Clinical relevance of sleep and vigilance in major depressive disorder: A review. Pimary Care Companion to Journal of Clinical Psychiatry. 2010; 12.