
Research Article
J Drug Discov Develop and Deliv. 2015; 2(1): 1013.
Physical Characteristics of Sixteen Non-Prescription Medications: Specific Gravity, Ph, Surface Tension, and Rheological Characteristics
Al-Achi A*, Kathuria A and Zahid Khan M
Department of Pharmacy and Health Sciences, University of Campbell, USA
*Corresponding author: Antoine Al-Achi, Department of Pharmacy and Health Sciences, University of Campbell, P.O. Box 1090, Buies Creek, NC 27506, USA
Received: August 25, 2015; Accepted: September 08, 2015; Published: September 29, 2015
Abstract
The physical characteristics of Over-The-Counter (OTC) medications serve as important parameters for the compounding pharmacist in order to prepare the prescription tailored to the patient’s needs. In this study, three containers of sixteen different OTC products were tested for their pH, surface tension, specific gravity, and their rheological behavior. All measurements were recorded at room temperature and in triplicates. The specific gravity was determined by the use of two different instruments (pycnometer and Densito 30 PX), and the results obtained from both methods were in good agreement with each other. All products were found to be denser than water except Witch Hazel USP Hamamelis Water which had a specific gravity of 0.98. Tustin DM cough syrup had the highest specific gravity of 1.26.pH of all products was found to be acidic except for DIgel Antacid and Antigay which showed basic pH of 8.07. Tustin DM cough syrup was found to be the most acidic with an average pH of 2.65. All products had an apparent surface tension value similar to or less than that of water. Pediatric Electrolyte had surface tension similar to that of water while Tustin DM Cough Syrup had the smallest value for surface tension (45.74 dynes/cm).Viscosities of all Newtonian and non-Newtonian products were measured by an Ostwald viscometer and a remoter, respectively, except for DI-gel Antacid and Anti gas was measured by a Brookfield Viscometer. Most of the Newtonian products had viscosity similar to water, except for Allergy Liquid, Children’s Day Time Cough and Cold Syrup, and Tustin DM Cough syrup which showed viscosity values of 3.23, 27.02, and 27.18 cPs, respectively. Children’s Day Time Cough and Cold Syrup had the highest kinematic viscosity of 22.41cSt, whereas Podia One Oral Therapy Rehydration had the lowest value of 1.02cSt. DI-gel Antacid and Antigay was found to exhibit thyrotrophic (pseudo plastic) behavior when subjected to stress. Triple Antibiotic Ointment and the toothpastes were tested using a remoter. Rheological parameters, namely compliance factor (Jo) and ground viscosity (?o), were determined from their respective rheograms. Triple Antibiotic Ointment was found with smallest compliance factor (Jo) value of 5.7 x 10-5 Pa-1, whereas the highest value was seen with Crest Toothpaste (3.12 x 10-3 Pa-1). The viscosity value of Max Block After sun Gel was found to be the highest (2.9 x 105Pa.s). The information obtained may serve as a resource for pharmacists while compounding medications for their patients.
Keywords: Over-The-Counter (OTC) medications; Viscosity determination; Rheological properties; Specific gravity
Introduction
Over-The-Counter (OTC) products are available to the public in the United States without prescription. In many countries, OTC drugs are selected by regulatory agencies to ensure that they are safe and effective when used without a physician’s advice. For the over-thecounter products consumer must be able to self-diagnose, self-treat, and self-manage [1]. In the United States, the manufacture and sale of OTC substances are regulated by the Food and Drug Administration.
The Active Pharmaceutical Ingredient (API) is considered to be the basis by which OTC drugs are regulated by the FDA regardless of the final product dosage form type [2]. The division in the FDA that oversees the OTC product is known as the Center for Drug Evaluation and Research (CDER). According to CDER, OTC drugs must be safe to use without physician supervision, properly labeled, and their benefits to the public outweigh their risk [3]. Unless the drug is Generally Regarded As Safe by the FDA (i.e., GRAS listed), the FDA requires New Drug Application (NDA) before it permits the product to be distributed in the United States, given that the product conforms to the Code of Federal Regulations (CFR) with all its labeling, warnings and doses [4].
A special class of OTC medications also exists whereas the patient is required to consult with a pharmacist prior to obtaining them. Examples of this class of medications are Schedule V controlled substances and emergency contraceptive pills. OTC class drugs may be extended in the future by the FDA to include some asthma medications and cholesterol controlling drugs [5]. Since January 2013, the FDA gave approval of several drugs to switch from prescription status to OTC class; the drugs were oxybutynin, triamcinolone actinide, esomeprazole magnesium, fluticasone propionate and, budesonide [6].
According to a study conducted in 2011, most Americans chose OTC medications for treating their common ailments such as headache, heartburn, allergies, and colds [7]. Pregnant women use 1.5 times more OTC products than medications prescribed by their physician. In The United States, around 40 % of the OTC drugs are used by the elderly patients [8]. OTC sales accrue $102 billion in value to US health care [9].
Commercially available OTC products serve as a good source of information to pharmacists to compound quality dosage forms if their physical and chemical characteristics are known. However, according to regulations governing the practice of pharmacy, pharmacists cannot exactly duplicate the commercially available formulations. Among the important physical characteristics of pharmaceutical products are specific gravity, pH, surface tension, and rheological behavior. It is known that these physical characteristics can influence the final product’s stability and palatability.
This study characterizes 16OTC products for their pH, surface tension, specific gravity, and rheological properties (i.e., flow characteristics, apparent viscosity, and compliance factor).
Material and Methods
Materials
Sixteen OTC products were selected for determining their physical characteristics (Table 1). All products (n = 3) were purchased from a local store in North Carolina.
Product
Distributor
Ingredients (Excluding additives such as coloring agents)
Pediatric Electrolyte
Greenbrier Internationals, Virginia
Dextrose, Potassium Citrate, Sodium Chloride, Sodium Citrate, Zinc Gluconate, Water
Lavoris Mouthwash
Evergreens Consumer Brands, Canada
Alcohol, Glycerin, Hydrogen Peroxide, Sodium Hexametaphosphate, Poloxamer 407, Polysorbate 80, Sodium Saccharin, Sodium Citrate, Xylitol, Citric Acid, Water
Citroma Magnesium Citrate
Vi – Jon, TN
Magnesium Citrate, Citric Acid, Lemon Oil, Polyethylene Glycol, Sodium Bicarbonate, Sodium Saccharin, Sucrose, Purified Water
Di-gel Antacid and Antigas
Health Products LLC, MD
Aluminum Hydroxide, Magnesium Hydroxide, Simethicone, Benzyl Alcohol, Butylparaben, Carboxymethylcellulose Sodium, Hypermallose, Microcrystalline Cellulose, Propylparaben, Saccharin Sodium, Sorbitol Solution, Purified Water
Allergy Liquid
Bio-Pharm Inc., Lewittown PA
Diphenhydramine HCl USP, Anhydrous Citric Acid, High Fructose Corn Syrup, Poloxamer 407, Sodium Benzoate, Sodium Chloride, Sodium Citrate, Sorbitol, Glycerin, Water
Witch Hazel USP Hamamelis Water
Vi – Jon, TN
Alcohol 14%v/v, Witch Hazel 86%v/v
Tussin DM Cough Syrup
Bio-Pharm Inc., Lewittown PA
Dextromethorphan HBr, Guaifenesin USP, Aspartame, Benzoic Acid, Glycerin, Menthol, Polyethylene Glycols, Water
Children's Day Time Cough and Cold
Bio-Pharm Inc., Lewittown PA
Dextromethorphan HBr, Phenyl HCl, Acesulfame Potassium, Anhydrous Citric Acid, Edetate Disodium, Maltitol, Propylene Glycol, Sodium Citrate, Alcohol, Benzoic Acid, Water
Pedia One Oral Therapy Rehydration
H:20 Innovations, LLC Ann Arbor, MI
Reverse Osmosis Water, Dextrose, Crystalline Fructose, Fruit and Vegetable Juice, Citric Acid, Ascorbic Acid, Stevia, Sodium Citrate, Potassium Citrate, Calcium Lactate, Gluconate, Niacinamide, D-calcium Pentothenate, Pyridoxine HCl , Cyanocobalamine,
Triple Antibiotic Ointment
Greenbrier Internationals, Virginia
Bacitracin Zinc, Neomycin Sulfate, Polymyxin B Sulfate, Petrolatum
Max Block Aftersun Gel
Greenbrier Internationals, Virginia
Water/EAU, Glycerine, Polysorbate 26, PEG-8, Sorbitol, Aloe Barbadensis Leaf Extract, Carbomer, Diazolidinyl Urea, Disodium EDTA, Tocopherol Acetate
Ice cold Topical Analgesic Gel
Dr. Sheffield’s
Menthol, Water, Alcohol, Isopropyl Alcohol, Methylchoroisothiazoline, bMethylsothiazoline, Sodium Hydroxide, CarbomarHomopolymer Type C
Colgate Toothpaste
Palmolive Company
Dicalcium Phosphate Dihydrate, Water Glycerin, Sodium Lauryl Sulfate, Cellulose Gum, Tetrasodium Pyrophosphate, Sodium Saccharin
Crest Tooth paste
Proctor and Gamble
Sodium Fluoride, Water, Sorbitol, Hydrated Silica, Sodium Acid Pyrophosphate, Sodium Lauryl Sulfate, Carboxymethylcellulose Sodium, Sodium Hydroxide, Saccharin Sodium, Xanthan gum, Mica, Titanium Dioxide
Aquafresh Toothpaste
Glaxo Smith Kline
Sodium fluoride (0.15% w/v fluoride ion), Water, hydrated silica, sorbitol, glycerin, pentasodium triphosphate, PEG-8, sodium lauryl sulfate, flavor, xantham gum, titanium
Pepsodent Toothpaste
Health Products, LLC
Calcium Carbonate, Water, Hydrated Silica, Sorbitol, Sorbitol, Sodium Lauryl Sulfate, Sodium Monofluorophosphate, Cellulose Gum, Sodium Silicate, Benzyl Alcohol, Potassium Nitrate, Triclosan, Sodium Saccharin
Table 1: Over-The-Counter Products Selected for Determinations of Physical Characteristics.
Methods
Specific gravity measurement: The specific gravity of product was determined using a pycnometer (Fisher Scientific, Pittsburgh, PA; Catalog No. 3-247Q). It is defined as the ratio of the weight of the product to the weight of the equal volume of water at a specified temperature. The empty weight of pycnometer was first obtained and then was tarred on an analytical weighing balance (Mettle-Toledo International, Inc., Columbus, OH; Model - Mettle AE163; Serial No. SC-133-05). An 11.5-mL volume of product was placed in a pycnometer and was weighed. This was repeated with an equal volume of distilled water. All measurements were done at room temperature and in triplicates. The specific gravity was also measured using Density 30 PX (Mettle-Toledo International, Inc., Columbus, OH). Samples were measured at room temperature in triplicates following the instructions specified by the manufacturer. The instrument was first calibrated with distilled water prior to measuring OTC samples. The specific gravity values were directly read from the instrument.
Measurement of pH: These measurements were performed on a calibrated pH meter (Denver Instrument; Model - UB - 10) at room temperature.
Surface tension measurement: Surface tension measurement was done using glass capillaries which were open on both the ends. A glass capillary was immersed in a 50-mL glass beaker containing 15 mL of the product. The capillary was positioned near the edge of the glass beaker so that the rise of liquid in capillary could be easily measured using a ruler. Distilled water was used as a reference standard and surface tension was calculated using the following expression:
γ1= (γ2d1h1) / (d2h2) (1)
where γ = surface tension (dynes/cm), d is the density (g/mL), and his the height of the liquid in centimeters above the surface of the liquid. The subscripts 1 and 2 refer to the test fluid and water, respectively.
Viscosity
Newtonian fluids: All Newtonian fluids were analyzed using Ostwald Viscometer (Barnstead International, Dubuque, IA; Type - Glass Capillary Viscometer; Item No.OST-001). The OTC sample or distilled water (9 mL), serving as reference, was introduced into the viscometer and the time (in minutes) needed for the sample to descent between two marks imprinted on the outer surface of the viscometer was recorded. All the readings were done in triplicates at room temperature. The viscosity of the fluid was determined from:
η1/ η2 = (ρ1/ρ2) (t1/t2) --------- (2)
where η is viscosity, ρ is the density, and t is time. The subscripts 1 and 2 refer to a reference standard (purified water) and test sample, respectively.
Viscosity and compliance measurement by rheometer: The viscosity of viscoelastic products was measured by a Rheometer (TA Instruments Ltd., Newcastle, DE; Model – Discovery HR – 2; Serial No. 5532-0381). The rheometer was set with specific parameters as shown in (Table 2) using Trios software (TA Instruments Ltd., Newcastle, DE; V 2.6) for performing step (transient) creep cycle. Small amount of sample was placed on the immovable bottom Peltier plate which is maintained at 25 0C throughout the run. The upper movable plate is lowered on the sample to trim gap and excess of sample was removed using a spatula. The movable plate is further lowered to set a gap of 1000.0 μm. Stress was applied for duration of 3 min and viscosity was calculated from the slope of the curve obtained in the linear region of graph, with Compliance on Y- axis and Step time on X-axis whereas compliance was calculated from Y intercept. All measurements were performed in triplicates.
Instrument geometry parameters
parallel plate
Specifications
20 mm
Peltier plate steel
997397
Diameter
20.0 mm
Gap
1000.0 μm
Loading gap
45000.0 μm
Trim gap
1050.0 μm
Minimum sample volume
0.314159 mL
Temperature
25 0C
Soak time
0 sec
Duration
180 sec
Stress
6.3662 Pa
Table 2: Specified Instrument Geometry Parameters for Rheometer.
Viscosity measurement by brook field viscometer: The viscosity of Di-gel Antacid & Antigens was measured by Brookfield viscometer in triplicates at room temperature. Spindle 1 (Brookfield Engineering Laboratory Inc., Middleborough, MA; LV Spindle set) was selected for testing the samples. Torque readings (%F) were read from the instrument at different shear rates (1, 2, 2.5, 4, 5, 10, 20 and 40 rpm). Apparent viscosity was calculated by plotting log shear rate (RPM) vs. log %F (torque). The mathematical expression representing this relationship is as follows:
log RPM = N log %F – log η’ (3)
where η’ is the apparent viscosity in centipoises which is calculated from the antilog value of they-intercept. From the slope of the line the parameter N is calculated which reflects the type of the rheological behavior the product exhibits (a value of 1 indicates Newtonian flow; for pseudo plastic and dilatants flown is greater than 1 and N is less than 1, respectively) [10].
Results and Discussion
The specific gravity measurements of all the products ranged between 0.920 to 1.262 (Table 4). Also, specific gravity measurements from both instruments (i.e. pycnometer and Density 30PX) showed good agreement with each other (Tables 3 and 4). The specific gravity of Witch Hazel USP Hamamelis Water was low because it contained 14 % alcohol by volume. On testing the pH of the products (Table 5), Di-gel Antacid &Antigens was the only product which had a basic pH (8.07). Tustin DM Cough Syrup was found to be the most acidic among all the products tested, having a pH of 2.65. The other syrup tested, namely Children’s Day Time Cough and Cold Syrup, had a pH of 3.45. This agrees with the commonly reported pH acidic values for pharmaceutical syrups. However, since these two syrup products were intended for the pediatric population, the low pH observed in the study might not be clinically ideal for this population. All the other products had pH ranging ranged from 4 to 5.
Product
Specific gravity mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
1.01±0.004
1.02±0.003
1.02±0.004
1.02±0.005
Lavoris Mouthwash
1.00±0.005
1.00±0.021
1.00±0.015
1.00±0.013
Citroma Magnesium Citrate
1.04±0.019
1.06±0.001
1.06±0.005
1.02±0.013
Di-gel, Antacid &Antigas
1.17±0.003
1.17±0.003
1.18±0.003
1.17±0.005
Allergy Liquid
1.10± 0.040
1.12±0.008
1.12±0.008
1.11±0.027
Witch Hazel USP Hamamelis Water
0.94±0.001
0.97±0.002
0.97±0.008
0.96±0.016
Tussin DM Cough Syrup
1.26±0.003
1.27±0.001
1.26±0.028
1.26±0.016
Children's Day Time Cough and Cold
1.21±0.017
1.22±0.002
1.20±0.019
1.21±0.014
Pedia One Oral Therapy Rehydration
1.00±0.007
1.00±0.021
1.00±0.015
1.00±0.013
Table 3: Specific Gravity of Products (Pycnometer).
zuct
Specific gravity mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
1.01±0.000
1.01± 0.000
1.01±0.000
1.01±0.000
Lavoris Mouthwash
1.01±0.000
1.01±0.000
1.013±0.000
1.01±0.000
Citroma Magnesium Citrate
1.05±0.000
1.06±0.004
1.06±0.000
1.06±0.003
Di-gel, Antacid &Antigas
1.16±0.000
1.15±0.003
1.16±0.001
1.16±0.005
Allergy Liquid
1.13± 0.00
1.13±0.000
1.13±0.000
1.13±0.004
Witch Hazel USP Hamamelis Water
0.98±0.000
0.98± 0.000
0.98± 0.000
0.98±0.000
Tussin DM Cough Syrup
1.25± 0.001
1.26±0.000
1.26±0.000
1.26±0.001
Children's Day Time Cough and Cold
1.21± 0.000
1.21±0.000
1.21±0.000
1.21±0.014
Pedia One Oral Therapy Rehydration
1.01±0.000
1.01±0.000
1.01±0.000
1.01±0.013
Table 4: Specific Gravity of Products (Densito 30 Px).
Product
pH mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
3.96±0.010
3.97±0.015
3.95±0.020
3.96±0.016
Lavoris Mouthwash
4.76±0.085
4.85±0.023
4.83±0.036
4.80±0.072
Citroma Magnesium Citrate
3.86±0.005
3.88±0.015
3.87±0.010
3.87±0.013
Di-gel, Antacid &Antigas
8.05±0.020
8.08±0.000
8.08±0.064
8.07±0.036
Allergy Liquid
4.75±0.020
4.79±0.034
4.85±0.106
4.80±0.073
Witch Hazel USP Hamamelis Water
4.25±0.080
4.23±0.005
3.92± 0.062
4.14±0.169
Tussin DM Cough Syrup
2.66± 0.015
2.64±0.045
2.66±0.041
2.66±0.073
Children's Day Time Cough and Cold
3.46± 0.015
3.44±0.062
3.47±0.010
3.46±0.035
Pedia One Oral Therapy Rehydration
3.99±0.023
3.99±0.010
3.97±0.011
3.99±0.016
Table 5: pH of Products.
Surface tension was found to be the highest for Pediatric Electrolyte having a value of 70.54 dynes/cm (which is similar to that of water) whereas Tustin DM Cough Syrup had the lowest value of 45.73 dynes/cm (Table 6). (Table 7) shows the viscosity of Newtonian products calculated from the data obtained with Ostwald viscometer. The lowest viscosity was 1.08 cPs (Pediatric Electrolyte) and the highest value was recorded for Children’s Day Time Cough and Cold Syrup (27.14 cPs) which was approximately 27 times greater than that of distilled water (due to its content of propylene glycol). All the other products had viscosity not exceeding 1.5 cPs, except for Allergy Liquid which had a value of 3.22 cPs; this may be due to its high fructose corn syrup content. (Table 8) shows kinematic viscosity of the products. The highest kinematic viscosity belonged to Children’s Day Time Cough and Cold Syrup (22.41cSt) while Pediatric Electrolyte had the lowest Kinematic viscosity value of 1.08cSt.
Product
Surface Tension mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
73.83±0.000
69.90± 4.261
68.90±4.262
70.54±3.891
Lavoris Mouthwash
59.04±0.000
61.53±4.262
59.84±0.000
60.14±2.398
Citroma Magnesium Citrate
66.42±4.424
66.67 ±4.441
69.39±0.000
67.50±3.444
Allergy Liquid
60.27±4.745
54.79±4.745
60.26±4.744
58.44±4.937
Witch Hazel USP Hamamelis Water
69.03±2.061
70.23±2.061
66.65±2.061
68.64±2.382
Tussin DM Cough Syrup
45.66±0.002
45.81±0.000
45.74±0.000
45.74±0.066
Children's Day Time Cough and Cold
43.85±0.000
49.71±5.064
52.76±0.000
48.77±4.668
Pedia One Oral Therapy Rehydration
66.42±0.000
61.51±4.260
51.66±0.000
59.87±6.849
Table 6: Surface Tension of Products by Capillary Rise Method (dynes/cm).
Product
Viscosity mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
1.18±0.235
1.22±0.004
1.03±0.002
1.08±0.013
Lavoris Mouthwash
1.21±0.007
1.77±0.012
1.18±0.004
1.19±0.015
Citroma Magnesium Citrate
1.46±0.007
1.50±0.056
1.51±0.018
1.49±0.0362
Allergy Liquid
3.19± 0.031
3.26±0.019
3.23±0.021
3.23±0.0365
Witch Hazel USP Hamamelis Water
1.43±0.006
1.46± 0.006
1.49± 0.002
1.46±0.027
Tussin DM Cough Syrup
26.25±0.804
28.34±0.878
26.83±0.696
27.14±1.094
Children's Day Time Cough and Cold
27.84±0.840
27.41±1.205
25.80±0.886
27.02±1.195
Pedia One Oral Therapy Rehydration
1.05±0.014
1.03±0.005
1.03±0.008
1.04±0.011
Table 7: Viscosity of Newtonian Products by Ostwald Viscometer (cPs).
Product
Kinematic Viscosity mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Pediatric Electrolyte
1.16±0.232
1.01± 0.004
1.02±0.002
1.06±0.013
Lavoris Mouthwash
1.19±0.007
1.60±0.012
1.61±0.004
1.17±0.015
Citroma Magnesium Citrate
1.38±0.007
1.41±0.056
1.43±0.018
1.41±0.0343
Allergy Liquid
2.82±0.031
2.89±0.019
2.86±0.021
2.86±0.032
Witch Hazel USP Hamamelis Water
1.45±0.006
1.49±0.006
1.52± 0.002
1.49±0.028
Tussin DM Cough Syrup
20.93±0.639
22.52±0.682
21.35±0.560
21.60±0.846
Children's Day Time Cough and Cold
23.12±0.709
22.75±0.997
21.36±0.734
22.41±1.012
Pedia One Oral Therapy Rehydration
1.04±0.014
1.02±0.005
1.03±0.008
1.03±0.011
Table 8: Kinematic Viscosity of Newtonian Products (cSt).
From the point of view of formulation development, the rheological characteristics of semisolid OTC products may be important for the stability of the API as well as considerations related to patient’s handling of the product (i.e., spread ability, wash ability, and extrusion). Other applications of rheological properties may include factors affecting the rate by which the API is released from the product. The rheological profiles of viscoelastic products obtained from rheometer are summarized in (Tables 8 and 9). Compliance factor (J0) for the products was obtained from the y-intercept of compliance (the quotient of strain and stress) vs. step time rheogram (creep curve) (Table 9). Viscosity was calculated by taking the inverse of the slope of the straight line segment of the curve. Crest Toothpaste showed the highest compliance factor (3.1x10-3 Pa-1) and Triple Antibiotic Ointment had the lowest compliance factor value (5.7x10-5Pa-1). Triple Antibiotic Ointment, the gels, and all the toothpastes exhibited characteristic viscoelastic properties, with Aqua fresh Toothpaste showed the highest ground viscosity average value of 291192.3 Pa. s (1 Pa. s = 1000 cPs). The two gel formulations had lower ground viscosity values than that of the toothpastes, and the ointment showed the least viscosity value of all the products tested (3910 Pa. s) (Table 10).
Product
Compliance *10-3mean±SD
Tube 1
Tube 2
Tube 3
Mean
Triple Antibiotic Ointment
0.05±0.001
0.06±0.001
0.06±0.006
0.06±0.003
MaxblockAftersun Gel
2.71±0.364
2.68±0.161
2.04±0.072
2.48±0.038
Ice Cold Topical Analgesic Gel
2.23±0.232
2.13±0.321
2.08±0.066
2.15±0.021
Colgate Toothpaste
0.73±0.058
0.68±0.058
0.97±0.153
0.79±0.006
Crest Toothpaste
2.50±0.173
3.13±0.153
3.73±0.252
3.12±0.056
Aquafresh Toothpaste
0.08±0.030
0.17±0.064
0.11±0.001
0.12±0.054
Pepsodent Toothpaste
0.16±0.019
0.13±0.056
0.16±0.061
0.15±0.047
Table 9: Compliance Factor of Non-Newtonian Products (Pa-1).
Product
Viscosity mean±SD
Tube 1
Tube 2
Tube 3
Mean
Triple Antibiotic Ointment
4234.33±403.827
3500.00±264.575
4000.00±1417.745
3910.00±816.265
MaxblockAftersun Gel
312500.00
247058.00
317833.33±13326.040
292000.00±816.265
Ice Cold Topical Analgesic Gel
380391.50±122010.568
145641.00±7977.57871
151283.00
226000.00±141461
Colgate Toothpaste
15088.67±6106.220
14555.67±6239.250
17300.00±12057.770
15648.11±7549.120
Crest Toothpaste
50133.33±14515.624
57600.00±11971.633
76666.67±2886.751
61500.00±15198.780
Aquafresh Toothpaste
306763.01±232717.491
173328.67±107219.218
393486.00±144243.024
291000.00±175610.980
Pepsodent Toothpaste
163515.00±136495.519
116840.33±71433.806
73736.67±52372.792
118000.00±90173.470
Table 10: Ground Viscosityof Non-Newtonian Products (Pa.s).
The viscosity of Di-gel Antacid and Antigens was determined by Brookfield viscometer (LV type). Log shear rate (RPM) was plotted against Logoff (torque) (Figures 1-3) and (Equation 3). From the linear fit obtained, the value of the y-intercept was negative. A positive value of less than one was calculated from taking the antilog of the y-intercept value. This indicates a pseudo plastic behavior (i.e., the viscosity of the product decreased with increasing the shearing rate). Similarly, the apparent viscosity of the product can be calculated from the slope of the line between log shear rates (RPM) vs. log %F (torque). For Di-gel Antacid and Antigens, a pseudo plastic behavior was observed with an apparent average viscosity of 56.40 cPs. It should be noted, however, that a great variability in the apparent viscosity among the bottles was observed (Table 11).
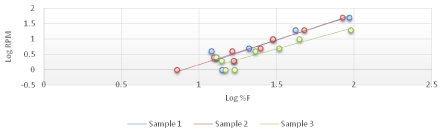
Figure 1: Log shear rate (RPM) vs. Log %F (torque) of bottle 1 of Di-gel Antacid and Antigens.
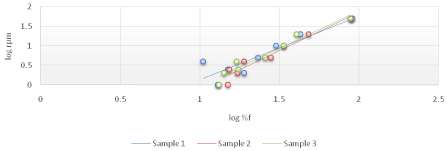
Figure 2: Log shear rate (RPM) vs. Log %F (torque) of bottle 2 of Di-gel Antacid and Antigens.
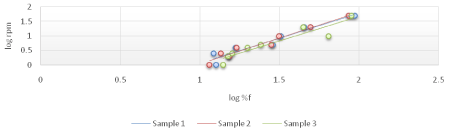
Figure 3: Log shear rate (RPM) vs. Log %F (torque) of bottle 3 of Di-gel Antacid and Antigens.
Product
Apparent Viscosity mean±SD
Bottle 1
Bottle 2
Bottle 3
Mean
Di-gel Antacid and Antigas
36.18±8.080
81.78±44.840
51.24±5.490
56.40±23.230
Table 11: Apparent Viscosity (cPs).
Conclusion
Over-The-Counter medications are popular in the United States. The physical characteristics of 16 OTC products determined in this study can serve as a good source of information for the compounding pharmacist to tailor his/her compounded dosage forms to match patient’s needs.
References
- Regulation of Regulation of Nonprescription Drug Products.
- Over-the-Counter Medicines: What's Right for You?
- Regulation of Nonprescription Drug Products.
- Over the Counter Medications.
- Ingredients & Dosages Transferred From Rx-to-OTC Status (or New OTC Approvals) by the Food an Drug Administration Since 1975: Consumer Healthcare Products Association. March 2015.
- Consumer Healthcare Product Association.
- Rubin J.D, Ferencz C, Loffredo C. Use of prescription and non-prescription drugs in pregnancy. J.Clin. Epidemiol. 1993; 46: 581- 589.
- Al-Achi A. Specific gravity determination of ten over-the- counter products. JPSI 2012; 5: 65.
- Al-Achi A. Shipp S. Physical Characteristics of Selected Over-the- Counter- Medications. IJPC 2005; 9: 75-81.
- Sinco P.J. Singh, Y. Rheology in Martin's physical pharmacy and pharmaceutical sciences: physical chemical and biopharmaceutical principles in the pharmaceutical sciences; Rice, Lippincott Williams & Wilkins: Baltimore, 2011; 469.