
Research Article
Austin J Emergency & Crit Care Med. 2017; 4(2): 1056.
Emergency Dialysis in End-Stage Renal Disease: Incidence and Characteristics in La Paz, Baja California Sur
Velazquez-Figueroa MA¹, Del Rio-Sanchez G², Segura-Trujillo M³, Ochoa MC4 and Ramirez- Leyva DH5*
¹Department of Emergency, General Hospital Zone #1 (IMSS), Baja California Delegation, Mexico
²Department of Family Medicine, General Hospital Zone #1 (IMSS), Baja California Delegation, Mexico
³Department of Nephrology, General Hospital Zone #1 (IMSS), Baja California Delegation, Mexico
4Department of Pediatrics, Regional General Hospital #1 (IMSS), Sonora Delegation, Sonora, Mexico
5Department of Family Medicine, Family Medicine Unit #1 (IMSS), Sonora Delegation, Sonora, Mexico
*Corresponding author: Ramirez Leyva Diego Hazael, Department of Family Medicine, Regional General Hospital #1 (IMSS), Sonora Delegation, Sonora, México, Colonia Centro, Cd. Obregon, Sonora, Mexico
Received: February 09, 2017; Accepted: March 14, 2017; Published: March 20, 2017
Abstract
Background: Chronic renal failure (CRF) is a highly prevalent disease in Mexico and the world, causing millions of deaths every year globally. Patients with CRF often require emergency dialysis due to electrolyte abnormalities and acid-base disorders.
Aim: So the purpose of this study is to determinate incidence and characteristics of patients with End-Stage Renal Disease (ESRD) and dialytic emergency in La Paz, Baja California Sur, Mexico.
Design and Setting: Comparative cross-sectional study.
Methods: In 60 patients with ESRD who needed emergency dialysis during January 2013 to December 2014 in General Hospital Zone #1, La Paz, Baja California Sur, Mexico; incidence of emergency dialysis was calculated. For each patient, demographic information, clinical features and biochemical parameters were obtained. There were two groups (man/woman) based on gender; it was used 80% statistical power and 95% interval confidence; for quantitative variables t-student or U-Mann-Whitney was used for statistical significance (p<0.05).
Results: Sixty patients (51.7% male and 48.3% female) were included. The average age was 56.2±17.1 years. The main presentation of dialytic emergency (DE) was uremic syndrome in 55% of cases; 20% pulmonary edema; 15% acidbase imbalance and 5% hyperkalemia. The clinical features were respiratory distress in 86.7%; 80% edema; 42% neurological symptoms and 18% electrocardiographic abnormalities. No gender differences in anthropometric and biochemical characteristics were found. The association between gender and clinical presentation of DE reported the following results: respiratory distress [p 0.017], electrocardiographic abnormalities [p 0.37], edema [p 0.03] and neurological symptoms [p 0.01].
Conclusion: DE is a rare alteration in La Paz, Mexico, with anincidence of 0.07%, primarily presented as syndrome uremic, pulmonary edema or metabolic acidosis.
Keywords: End-Stage Renal Disease; Emergency Dialysis; Nephrology
Introduction
According to international guidelines of Kidney Disease: Improving Global Outcomes (KDIGO, 2012); chronic renal failure (CRF) is defined as those abnormalities of kidney structure or function, present for more than 3 months with implications for health. CRF is classified according to cause, category of glomerular filtration rate (GFR) and category of albuminuria [1]. The estimated prevalence of CRF is 16.8% worldwide. CRF can progress to end-stage renal disease (ESRD), which requires dialysis or transplantation. However, many patients cannot undergo such therapies because of its high cost [2- 3].Complications of CRF include dialytic emergency, anemia, renal osteodystrophy and malnutrition, among others [4].
CRF is a highly prevalent pathology that affects people of all races, nationalities, age, gender and economic level. Low socioeconomic status and poor access to health services contribute to inequality in health care and exacerbate negative effects of genetic or biological predisposition [5-6]. It is precisely the people with little or no access to health services who are at greater risk for complications of CRF [6].
According to annual report of the United States Renal Data System, main causes of end-stage renal failure in patients with CRF are diabetes (153 cases per million inhabitants in 2009), arterial hypertension (99 cases per million inhabitants) and glomerulonephritis (23 cases per million inhabitants). Cardiovascular disease is also an important cause; however, about 28% of patients with clinically significant CRF (stage 3 or higher) are not diabetic or hypertensive, especially those older than 65 years. In developing countries, diabetes and hypertension are currently the leading causes of CRF with a prevalence of 30% and 21% respectively, but glomerulonephritis and CRF of unknown origin are responsible for a greater proportion of ESRD, especially in young patients [3,7].
In Mexico, CRF is one of main causes of morbidity and mortality and one of main causes of hospitalization in emergency departments. CRF is considered a catastrophic disease due to increasing number of cases, high investment costs, limited infrastructure and human resources, late detection and high morbidity and mortality rates in substitution programs. In Mexico, prevalence and incidence of patients with CRF is unknown and the precise number of patients in any of its stages, age groups and gender most affected is unknown. An incidence of 377 cases per million inhabitants and a prevalence of 1,142 per million is estimated. In Mexico there are about 52,000 patients on dialysis, of which 80% are treated at the Mexican Social Security Institute (IMSS) [8].
The pathophysiology of CRF involves two types of damage mechanisms: mechanisms of damage initiation (genetic abnormalities, deposit of immune complexes and inflammation in certain types of glomerulonephritis, exposure to toxic and interstitial tubule diseases) and progressive mechanisms, which involve hyperfiltration and hypertrophy of viable nephrons as consequence of long-term reduction of renal mass. The reaction to reduction in number of nephrons is determined by vasoactive hormones, cytokines and growth factors. Eventually, these short-term adaptations of hypertrophy and hyperfiltration result in an increased pressure and flow, predisposing to distortion of glomerular architecture and damage of remaining nephrons. The increased intrarenal activity of renin-angiotensinaldosterone axis seems contribute to initial adaptive hyperfiltration, hypertrophy and sclerosis. In sclerosis, transforming growth factor β is involved (TGF-β). This process explains why a reduction in renal mass may lead a progressive decrease in renal function over the years [5].
Diagnosis of CRF should be suspected in patients with risk factors and confirmed by estimation of glomerular filtration rate, followed by assessment of renal injury [1]. Serum creatinine level should not be used as the only test to evaluate renal function; the best tool is calculation of GFR. Calculation of GFR from creatinine clearance (measurement of creatinine concentration in serum and 24-hour urine) has drawbacks, such as overestimation of GFR and 24-hour urine collection techniques for both patients as for laboratories. For a more accurate estimate it is recommended the use of some equations to calculate GFR, which consider various factors such as serum creatinine concentration, age, sex and ethnicity [9]. The most used equations are Modification of Diet in Renal Disease (MDRD- 4, MDRD-6), Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) and Cockcroft-Gault (C-G) [9].
Dialytic emergency is diagnosed when the patient presents intractable metabolic acidosis, hyperkalemia with electrocardiographic alterations or fluid overload with pulmonary edema or uremia [4]. Although CRF is a very frequent and highly relevant condition for public health, no reports of frequency of dialysis were found in patients with CRF.Based on the above, main objective of this study was to determinate incidence and characteristics of patients with ESRD and dialytic emergency in La, Paz, Baja California Sur, Mexico.
Materials and Methods
A comparative, cross-sectional study was carried out in the General Hospital Zone #1 of the Mexican Institute of Social Security (IMSS) in La Paz, Baja California Sur, Mexico, from January 2013 to December 2014. All medical records of patients in emergency department were reviewed and information was collected of patients that met the following inclusion criteria: any gender, older than 5 years, diagnosis of ESRD and clinical or biochemical data of ESRD with dialytic emergency; were eliminated those who did not have complete information (clinical, biochemical or medical records). The following data were obtained directly from the medical records or patients: age, gender, diagnosis of ESRD, occupation,educational stages, time from onset of ESRD(considered the approximate date of diagnosis), clinical presentation of dialytic emergency, weight, height, body mass index (BMI=weight/height2), biochemical parameters (urea, creatinine, microalbuminuria, serum sodium, serum calcium, serum potassium, glycosylated hemoglobin), baseline vital signs: blood pressure, heart rate, respiratory rate, electrocardiogram and gasometry interpretation, glomerular filtration rate (CDK-EPI) and comorbidities or chronic degenerative diseases as Diabetes Mellitus (DM), arterial hypertension (HA) and glomerulonephritis (GMN).
The data obtained was integrated into data collection sheets and analyzed using the SPSS program version 20 in Spanish, where we applied descriptive statistics; for qualitative variables frequencies and percentages were used and for quantitative variables mean and standard deviation were used. For bivariate analysis was considered statistically significant a p <0.05, with a 95% confidence interval, for quantitative variables t-student or U-Mann-Whitney was used. The Protocol was authorized by the Local Committee of Research and Ethics in Health Research from the General Hospital Zone#1, where the study took place.
Results
Patients and medical records of emergency department in General Hospital Zone #1 of La Paz, Baja California Sur, Mexico, were reviewed.A total of 60 patients with dialytic emergency were identified between January 2013 and December 2014, of whom 31 were female and 29 were male (51.7% vs. 48.3%, respectively). The mean age was 56.2±17.1 years, minimum age 7 years and maximum 86 years. Prevalence of dialytic emergency by age group was (Figure 1): 3.3% in patients under 16 years of age; 6.6% in 17-30 years; 15% in 31-45 years; 26.7% in 46- 60 years; 36.7% in 61-75 years and 11.7% in those over 76 years. The overall incidence of dialytic emergence was 0.07%.
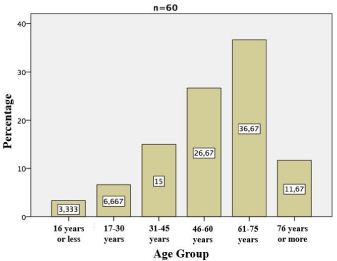
Figure 1: Frequency of dialytic emergency according to age group.
The mean time of ESRD diagnosis was 7.9±3.1 years, minimum 1 year and maximum 13 years. Etiology of chronic renal failure was diabetes mellitus in 5% of cases, arterial hypertension in 13.3% and a combination of diabetes and hypertension in 81.7% of cases. The clinical presentation of dialytic emergency was: uremic syndrome in 55% of cases, pulmonary edema 20%, acid-base imbalance 15% and hyperkalemia 5%. Metabolic acidosis was the cause of 1.67% of dialytic emergency cases, 1.67% a combination of pulmonary edema plus hyperkalemia and pulmonary edema plus acid-base imbalance plus pulmonary edema was responsible for 1.67% of cases.
The clinical characteristics of dialytic emergency were: respiratory distress in 86.7% of patients, edema 80%, neurological symptoms 41.7% and electrocardiographic alterations 18.3% of cases. Average anthropometric characteristics and baseline vital signs were as follows (Table 1): weight 75.4±17.4 kilograms, height 1.6±0.1 meters, BMI 28.9±5.9 kg/m2, respiratory rate 22.4±4.9 respirations per minute, heart rate 81.7±15.2 beats per minute, systolic blood pressure130.4±40.5 mmHg and diastolic blood pressure 78.5±18.4 mmHg. The average of measured biochemical parameters are described below (Table 2): urea 161.4±60.0 mg/dL, creatinine7.3±3.9 mg/dL, microalbuminuria 2.9±0.5 mg/L, glycosylated hemoglobin 9.5±1.8 %, serum potassium 5.4±1.2 mEq/L, serum sodium 134.1±18.6 mEq/L and serum calcium 8.5±3.7 mEq/L. Glomerular filtration rate was 7.7±3.9 ml/hr/1.73m2, minimum 2.0 and maximum 19.0 mL/min/1.73m2.
Variable
N
µ
SD
Minimum
Maximum
Weight (kg)
60
75.4
±17.4
47
120
Height (mt)
60
1.60
±0.1
1.4
1.8
Body Mass Index
60
28.9
±5.9
20.9
44.1
Respiratory rate
60
22.4
±4.9
8.0
35.0
Heart rate
60
81.7
±15.2
40
120
Systolic blood pressure
60
130.4
±32.1
70
234
Diastolic blood pressure
60
78.5
±18.4
40
124
N: Frequency, µ: Mean, SD: Standard Deviation, kg: kilograms, mt: meters.
Table 1: Anthropometric characteristics and baseline vital signs of patients with ESRD.
Variable
N
µ
SD
Minimum
Maximum
Urea (mg/dl)
60
161.4
±60
8
284
Creatinine (mg/dl)
60
7.6
±3.9
1.8
16.8
Microalbuminuria (mg/dl)
60
2.9
±0.5
1.5
4.9
Glomerular Filtration Rate (ml/min/1.73m2)
60
7.7
±3.9
2
19
Glycosylated hemoglobin (%)
60
9.5
±1.8
4.9
13.2
Potassium (meq/L)
60
5.4
±1.2
2.6
7.9
Sodium (meq/L)
60
134.1
±18.6
7.9
147
Calcium (meq/L)
60
8.5
±3.7
2.8
19
N: Frequency, µ: Mean, SD: Standard Deviation, mg/dl: milligrams/deciliters, ml/min/1.73m2: milliliter/minute/1.73m2, %: percentage, meq/L: millequivalent/Liter.
Table 2: Biochemical characteristics of patients with ESRD.
The following results were obtained for the comparison between clinical characteristics and gender (Table 3): age [p 0.36], time of diagnosis [p 0.19], weight[p 0.76], height [p 1.0], BMI [p 0.11], respiratory rate [p 0.39],heart rate [p 0.65], systolic blood pressure [p 0.41], diastolic blood pressure [p 0.54], urea [p 0.58], creatinine [p 0.88], microalbuminuria [p 0.37], glomerular filtration rate [p 0.88], glycosylated hemoglobin [p 0.74], sodium [p 0.37], potassium [p 0.62] and calcium [p 0.70]. When comparing clinical presentation of ED and gender (Table 4) we found that respiratory distress as a symptom of dialytic emergency was more frequent in female patients than in male patients [96.8% vs 75.9%, p 0.01], as well as edema [90.3% vs 69%, p 0.03] and electrocardiographic alterations [22.6% vs 13.8%, p 0.37]; but, neurological alterations were more frequent in men than in women [58.6% vs 25.8%, p 0.01].
Characteristic
Gender
µ
SD
p
Age
(years)
Woman
58.1
±16.5
0.36
Man
54.1
±17.9
Time of diagnosis
(years)
Woman
8.4
±2.4
0.19
Man
7.4
±3.6
Weight
(kilograms)
Woman
74.7
±18.8
0.76
Man
76.2
±16.3
Height
(meters)
Woman
1.6
±0.1
1.0
Man
1.6
±0.1
Body Mass Index
(kg/m2)
Woman
30.2
±6.5
0.11
Man
27.6
±5.0
Respiratory rate
(rpm)
Woman
22.9
±4.5
0.39
Man
21.8
±5.3
Heart rate
(bpm)
Woman
82.5
±15.6
0.65
Man
80.8
±15.1
Systolic blood pressure (mmHg)
Woman
140.5
±35.1
0.41
Man
133.7
±28.9
Diastolic blood pressure (mmHg)
Woman
80.6
±19.6
0.54
Man
77.8
±15.4
Urea
(mg/dl)
Woman
165.6
±68.6
0.58
Man
156.9
±50.1
Creatinine
(mg/dl)
Woman
7.5
±4.0
0.88
Man
7.6
±3.9
Microalbuminuria
(mg/dl)
Woman
2.9
±0.6
0.37
Man
2.8
±0.4
Glomerular Filtration Rate (ml/min/1.73m2)
Woman
7.7
±3.7
0.88
Man
7.8
±4.2
Glycosylated hemoglobin(%)
Woman
9.4
±1.8
0.74
Man
9.5
±1.8
Potassium
(meq/L)
Woman
5.5
±1.4
0.62
Man
5.3
±1.1
Sodium
(meq/L)
Woman
136.3
±6.0
0.37
Man
131.8
±25.7
Calcium
(meq/L)
Woman
8.1
±0.9
0.70
Man
8.9
±5.4
µ: Mean, SD: Standard Deviation, p: t-student (normal distribution) or U-Mann-Whitney (abnormal distribution), rpm: respirations per minute, bpm: beats per minute, mmHg: millimeters of mercury, mg/dl: milligrams/deciliters, ml/min/1.73m2: milliliter/minute/1.73m2, %: percentage, meq/L: millequivalent/Liter.
Table 3: Differences between clinical and biochemical characteristics according to gender in ESRD.
Clinical presentation
Gender
p
Woman (%)
Man (%)
Dyspnea
96.8
75.9
0.01
Electrocardiographic alterations
22.6
13.8
0.37
Neurological disorders
25.8
58.6
0.01
Edema
90.3
69.0
0.03
%: percentage, p: t-student (normal distribution) or U-Mann-Whitney (abnormal distribution).
Table 4: Differences between clinical presentations according to gender in ESRD.
Discussion
In research study clinical presentation of dialytic emergency were uremic syndrome, pulmonary edema, acid-base imbalance and hyperkalemia. It has been reported that uremic syndrome occurs when glomerular filtration rate is reduced to less than 10mL/min/1.73m2 in non-diabetic patients and when it is less than 15mL/min/1.73 m2 in diabetic patients [10-11]. In contrast to the present study in which a low frequency of hyperkalemia (5%) was found; Einhorn et al (2009), found a hyperkalemia global incidence of 25.6% in patients with renal failure and in patients with end-stage renal disease (stage 5) incidence of hyperkalemia was 56.7% [12].
The main danger of hyperkalemia is electrocardiographic abnormalities [13-14], in fact, in present study 81.9% of electrocardiographic abnormalities were present in patients with hyperkalemia and only 18.2% in patients with normokalemia. It has been reported that incidence of metabolic acidosis in patients with ESRD is around 19% and may reach 80% in patients with GFR<20 mL/min/1.73 m², in present study only 15% of patients presented metabolic acidosis. Negative effects of metabolic acidosis on ESRD include: worsening renal osteodystrophy, growth retardation in children, rhabdomyolysis, decreased albumin synthesis and increased mortality [15-16].
Hydric overload, with or without pulmonary edema is common in patients with dialytic emergency; in fact, edema and respiratory distress were the most frequent clinical manifestations of DE in present study. These complications typically occur when GFR falls below 10-15ml/min/1.73m² [17-18]. The etiology of renal failure in all patients included in this study was systemic arterial hypertension, diabetes or a combination of both. This finding is similar to that reported by worldwide literature which reports that main causes of ESRD are systemic arterial hypertension and diabetes mellitus [19].
Conclusion
There is no specific national or global information on dialytic emergencies with which to compare the outcome of this study since most of efforts are focused on preventing progression of this disease. The incidence of this complication is low in this region of Mexico with 0.07% and increases with age, especially in those over 30 years. Main manifestations were dyspnea, edema, electrocardiographic changes and neurological alterations, which represent the basis for diagnosis of this type of emergency that can endanger the life of our patient. The present study can be considered as a starting point for future research on end-stage renal disease, its characteristics and treatment.
References
- KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International. 2013; 3: 1–150.
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013; 382: 260-272.
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011; 80: 1258-1270.
- Rozo Uribe R, D’Achiardi Rey R. Nephrology. Practices and procedures. Clinical practice guidelines. Volume VII, 1st edition. Bogota: Latin American Medical Editions. 2003; 126–139.
- Unruh M.L. (2009). Chapter 87.End-Stage Renal Disease. In Halter J.B., Ouslander J.G., Tinetti M.E., Studenski S, High K.P., Asthana S (Eds), Hazzard's Geriatric Medicine and Gerontology, 6e. 2009.
- Garcia-Garcia G, Jha V. World Kidney Day Steering Committee. Chronic kidney disease in disadvantaged populations. Braz J Med Biol Res. 2015; 8: 3-6.
- Collins AJ, Foley RN, Herzog C. United States Renal Data System 2008 Annual Data Report. Am J Kidney Dis. 2009; 53: S1–S374.
- Méndez-Durán A, Méndez-Bueno JF, Tapia-Yáez T, Muoz-Montes A, Aguilar-Sánchez L. Epidemiology of chronic renal failure in Mexico. Dialisis y Transplantes. 2010; 3: 7–11.
- Martínez-Castelao A, Górriz JL, Bover J, Segura-de la Morena J, Cebollada J, Escalada J, et al. Consensus document for the detection and management of chronic kidney disease. Aten Primaria. 2014; 34: 243-262.
- May RC, Mitch WE. Pathophysiology of uremia. In: Brenner BM, ed. Brenner & Rector's The Kidney. Vol 2. 5th ed. Philadelphia, Pa: WB Saunders; 1996: 2148-2169.
- Vanholder R. The Uremic Syndrome. In: Greenberg A, ed. Primer on Kidney Disease. 2nd ed. San Diego, Calif: Academic Press; 1998: 403-407.
- Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009; 169: 1156-1162.
- Lehnhardt A, Kemper MJ. Pathogenesis. Diagnosis and management of hyperkalemia. PediatrNephrol. 2011; 26: 377-384.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am SocNephrol. 2008; 3: 324–330.
- Ori Y, Zingerman B, Bergman M, Bessler H, Salman H. The effect of sodium bicarbonate on cytokine secretion in CKD patients with metabolic acidosis. Biomed Pharmacother. 2015; 71: 98-101.
- Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005; 45: 978-993.
- Kalantar-Zadeh K, Regidor DL, Kovesdy CP et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009; 119: 671–679.
- Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010; 56: 512–517.
- Unruh ML. Chapter 87. End-Stage Renal Disease. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S. eds. Hazzard's Geriatric Medicine and Gerontology, 6e. New York, NY: McGraw-Hill; 2009. P. 675-682.