Research Article
Austin J Endocrinol Diabetes. 2014;1(3): 1012.
Lingonberry (Vaccinium vitis-Idaea L) Mobilizes L6 Muscle GLUT4 Transporters and Exerts Anti-Obesity and Antidiabetic Effects in vivo
Hoda M Eid1,2,5, Antoine Brault1,2, Meriem Ouchfoun1,2, Farah Thong4, Diane Vallerand1,2, Lina Musallam1,2, John T Arnason2,3, Gary Sweeney4 and Pierre S Haddad1,2*
1Natural Health Products and Metabolic Diseases Laboratory, Dept. of Pharmacology, Université de Montrèal, Montreal, Quebec, Canada
2Canadian Institutes of Health Research Team in Aboriginal Antidiabetic Medicines and Montreal Diabetes Research Center, Montreal, Quebec, Canada
3Phytochemistry, Medicinal Plant and Ethnopharmacology Laboratory, Dept. of Biology, University of Ottawa, Ottawa, Ontario, Canada
4Dept. of Biology, York University, Toronto, Ontario, Canada
5Department of Pharmacognosy, University of Beni-seuf, Beni-seuf, Egypt
*Corresponding author: Pierre S. Haddad, Department of Pharmacology, Université de Montréal, P.O. Box 6128, Centre-Ville Station, Montreal, Quebec, H3C 3J7 Canada,
Received: February 10, 2014; Accepted: March 06, 2014; Published: March 13, 2014
Abstract
Lingonberry (Vaccinium vitis-idaea L.) is an important part of Scandinavian diet. It is also popular in some parts of Europe and North America, and is used to produce confectionary and food products. This plant has been identified among species used by the Cree of EeyouIstchee (northern Quebec) to treat symptoms of diabetes.
In a previous study, the ethanol extract of V. vitis berries enhanced glucose uptake in C2C12 muscle cells through stimulation of AMP–activated protein kinase (AMPK) pathway. In this study, we firstly investigated the effect of this product on the translocation of insulin–sensitive glucose transporters GLUT4 in L6-GLUT4myc skeletal muscle cells. V. vitis extract (200 μg⁄ml, 18h) significantly increased glucose uptake and induced GLUT4 translocation to the cell membrane of L6 cells through an insulin–independent mechanism involving AMPK.
Secondly, we carried out in vivo experiment to validate its antidiabetic effect. The extract was administered to diabetic KKAy mice for 10 days. V. vitis decreased glycaemia, cumulative food intake and body weight. Moreover, V. vitis tended to increase skeletal muscle GLUT4 expression and attenuated hepatic statuses.
These results demonstrate that V. vitis berries represent a promising avenue for the culturally adapted management of obesity and diabetes in Canadian aboriginals.
Keywords: Type 2 diabetes mellitus; Obesity; GLUT4; V. vitis; lingonberry; KKAy mice; AMPK.
Introduction
Several members of the genus Vaccinium bear edible berries; many of them are reputed for their antidiabetic activity. European blueberry or bilberry (V. myrtillus L.) were widely used in Europe to treat diabetes prior to the discovery of insulin [1]. In one study, unsweetened cranberry juice (V. macrocarpon Ait.) helped to lower blood glucose levels in patients with type 2 diabetes [2]. In addition,Canadian blueberry (V. angustofolium Ait.) has been observed to exhibit antidiabetic activities in cultured skeletal muscle [3]. Finally, a recent study has shown that the bio–transformed blueberry juice incorporated in the drinking water reduced hyperglycemia of diabetic KKAy mice [4].
Aboriginal populations are particularly at risk for developing type 2 diabetes mellitus and its complications. In Canada, the prevalence of diabetes for these populations is at least three times higher than that of the general population and is expected to increase three–fold over the next 20 years [5]. Lingonberry (Vaccinium vitis–idaea L.), known also as mountain cranberry or partridgeberry, is consumed by the Cree communities of EeyouIstchee (CEI, Eastern James Bay region of the Canadian province of Quebec) not only as food but also as medicine to treat symptoms of diabetes including frequent urination [6,7]. Our research team identified his plant during a previous bioactivity screening study [8], as part of a project aiming to provide culturally relevant alternative treatment options for Cree diabetics, whose disease prevalence is among the highest in Canada.
In our previous study, V. vitis was found to increase glucose transport in muscle cells through the activation of AMPK as a response to metabolic stress resulting from a non–toxic disruption of mitochondrial energy transduction [9]. The present study was carried out firstly to determine whether V. vitis increases GLUT4 translocation in skeletal muscle cells as suspected from enhanced glucose transport. We selected L6 myocytes because they express more GLUT4 proteins than our previous C2C12 cellular model and because tools exist to better ascertain GLUT4 translocation to the plasma membrane. Secondly, our objective was to confirm the antidiabetic activity of V. vitis in an in vivo model of type 2 diabetes. KKAy mice are a cross between glucose–intolerant black KK female mice and yellow obese Ay male mice. They are characterized by hyperphagia, insulin resistance, hyperinsulinemia, diabetes, dyslipidemia and hypertension. Therefore, KKAy mice are an excellent model for type 2 diabetes induced by obesity [10]. This model was thus selected to evaluate the in vivo antidiabetic activity of V. vitis.
Materials and Methods
Plant material and extraction
Berries of V. vitis were collected in the Eastern James Bay region, QC, Canada, and kept at −20°C until use. Botanical identity was confirmed by Dr. Alain Currier (Institute de recherché en biologie végétale, Université de Montréal), plant taxonomist on our Team, and voucher specimens were deposited at the Montreal Botanical Garden herbarium (voucher # Whap04–21).
In total 800 g of the berries were freeze–dried (Super Modulo freeze dryer; Thermo Fisher, Ottawa, Ont, Canada) to yield 114 g of dry material. The dry material was then extracted three times for 24 h with ten volumes of 80% ethanol on a mechanical shaker and then filtered under vacuum using Whitman 1 paper. The supernatants were combined and dried using a rotary evaporator (RE 500; Yamato Scientific, Tokyo, Japan) followed by lyophilization.
Cell culture
Rat L6 skeletal muscle cells were grown in minimum essential medium alpha (α–MEM) supplemented with 10% (v⁄v) fetal bovine serum (FBS) in a 5% CO2 at 37°C and used as myoblasts when fully confluent. For differentiation into my tubes, cells were switched to a medium containing 2% FBS for 5–7 days. Cells transfected to stably overexpress GLUT4 harboring mycepitope on the first exofacial loop of the transporter (L6 GLUT4myc cells) were provided by Dr Amira Klip (The Hospital for Sick Children, Toronto, ON, Canada).
Measurement of glucose uptake
L6–GLUT4myccells were cultured in 12–well plates and were used after 5–7 days of differentiation. The cells were serum–starved for 4 h before incubation with the maximum non–toxic concentration of V. vitis (200 μg⁄ml) for 18 h or insulin (100 nM, 20 min). Cells were incubated in transport solution [140 mM NaCl, 20 mM HEPES–Na, 2.5 mM MgSO4, 1 mM CaCl2, 5 mMKCl, 10 μM 2–Deoxy–Glucose and 0.5 μCi⁄ml 2–deoxy–D–[3H] glucose (pH 7.4)] for 5 min at room temperature. Cells were then lysed with 1 M KOH and aliquots were transferred to scintillation vials for 3H radioactivity counting and expressed as fold increase over control. Nonspecific uptake was measured in the presence of cytochalasin B (10 μM) and was subtracted from all values.
Determination of cell surface GLUT4 (OPD assay)
Levels of GLUT4 mycat the cell surface were measured by an antibody–coupled colorimetric assay [11]. Briefly, L6 myoblasts were cultured in 24–well plates until confluence and serum–starved for 4 h before incubation with either V. vitis (200 μg⁄ml) for 18 h or insulin (100 nM) for 20 min. Cells were then quickly washed in ice–cold PBS and incubated with an anti–c–mycantibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 60 min at 4°C. After that, cells were washed and fixed in 3% para– formaldehyd for 3 min on ice. To neutralize the fixative, cells were incubated with 10 mM glycine in ice–cold PBS for 10 min, and then blocked in 5% goat serum for 30 min. Horseradish peroxides (HRP)–conjugated goat anti–rabbit IgG was then applied for 60 min at 4°C (1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA). Cells were washed five times with ice–cold PBS and incubated with O–phenylenediamine dihydrochloride (OPD) reagent (1 ml⁄well) (Sigma–Aldrich, St. Louis,MO, USA) at room temperature for 30 min. To stop the reaction, 0.25 ml of 3 M HCl was added to each well. The supernatant was collected and its absorbance was measured at 492 nm. Absorbance associated with nonspecific binding (primary antibody omitted) was used as a blank.
Animals and in vivo experimental protocols
Study #1: Effect of V. vitis on diabetic KKAy mice. KKAy mice were derived from an in–house colony established using breeding pairs obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice weighing 33–42 g were housed individually and kept for 1 weekon a 12 h light–dark cycle in a temperature controlled chamber and provided with a regular laboratory chow and water ad libitum. The animals were divided into two groups containing seven mice each, as follows: Group 1 diabetic mice, average body weight of 36.7± 0.89,received drinking tap water and served as controls; Group 2 diabetic mice, average body weight of 37.8± 1.21 were administered with 1% V. vitis in drinking water (equivalent to a dose of 4 g⁄ kg of crude berry extract on the first day of treatment and 1.33 g⁄kg thereafter, due to a drop in fluid intake until the end of the experiment). The dose was chosen on the basis of preliminary experiments. In addition, bilberry, another vaccininium species ( ) reputed to possess antidiabetic activities, was administered to rats at a similar dose of 3 g⁄kg⁄day [12]. During the ten days of treatment, body weight as well as food and fluid intake were determined on a daily basis. The non–fasting blood glucose concentration was measured daily using an Accu–Chek glucometer (Roche, Montreal, QC, Canada) by collecting blood from the tip of the tail vein. On the last day of treatment, the mice were anaesthetized, sacrificed and organs such as liver, skeletal muscle, kidney, epididymal fat pad, abdominal fat pad and dorsal fat pad were immediately removed and stored in a −80° C freezer until used. All experimental protocols were approved by the animal experimentation ethics committee of the University of Montreal and carried out in full respect of the guidelines from the Canadian Council for the Care and Protection of Animals.
Study #2: Pair–feeding effect in KKAy diabetic mice
Pair feeding was employed in order to investigate to what extent the blood glucose–lowering effect observed with V. vitis in study #1 could be attributed to the observed reduction in food intake. Animals were allocated into two groups containing seven mice each as follows: Group 1 diabetic mice, weighed 33.74 ± 0.85, were administeredwith 1% V. vitis in drinking water; Group 2 diabetic mice, weighed 33.16 ± 1.55, received drinking water and were pair–fed to group 1 mice. Pair feeding was carried out by measuring the food intake of the ad libitum–fed V. vitis treated mice every 24 h and presenting this amount of food to the pair–fed treated mice with a one–day delay.Food consumption, fluid intake, body weight and blood glucose were recorded daily. At the end of the study, the mice were sacrificed, blood samples were obtained and tissues were harvested as described for study #1.
Study #3: normal C57BL⁄6J mice
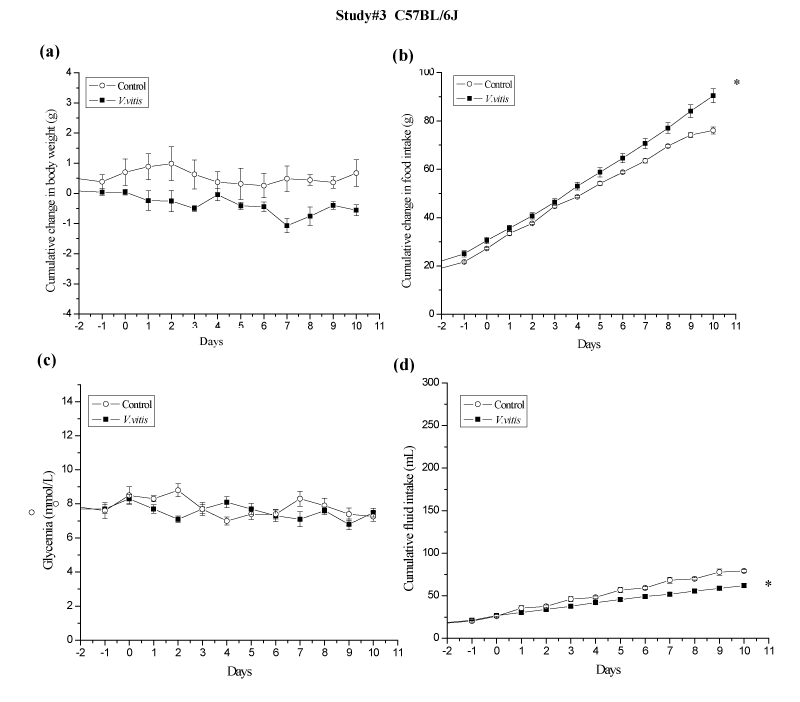
To study the effect on V. vitis on blood glucose levels and food intake in normal animals, normal C57BL⁄6J mice were housed as described before and randomly divided into two groups containing seven mice. Group 1 mice (average body weight of 28.8± 0.49) received drinking water and served as control; Group 2 mice (averagebody weight of 28.92 ± 0.41) were administered with 1% V. vitis in drinking water. Both groups were fed regular laboratory chow ad libitum. The experimental protocol lasted for 10 days and was performed as described for study #1.
At the end of each study, the mice were anesthetized using 50 mg⁄kg pentobarbital intraperitoneally and then sacrificed by exsanguinations via the inferior vena cava.
Blood parameters
Plasma insulin and adiponectin levels were determined using radioimmunoassay kits (Linco Research, St–Charles, MO, USA). The levels of serum triglycerides, cholesterol, HDL, LDL, creatinine, alkaline phosphates, AST (Aspirate aminotransferase), ALT (Alanine aminotransferase) and LDH (lactate dehydrogenize) were measured by the Department of Biochemistry of Sainte–Justine’s Children Hospital (Montreal, QC, Canada).
Western blot for proteins involved in glucose and lipid metabolism
The effects of the plant extract on insulin and AMPK signaling pathways in L6 muscle cells were assessed by western immunoblot analysis. Differentiated L6 cells (5–7 day) cultured in 6–well plates were treated with plant extract or vehicle alone (DMSO) for 18 h.Twenty minutes prior to the end of the treatment, insulin (100 nM) or aminoimidazole carboxamide ribonucleotide (AICAR; 1 mM) were added to some vehicle–treated wells as positive controls. For ACC and GLUT4 western blot analysis, samples of muscle of KKAy mice from studies #1 & 2 were used. L6 cells and muscle tissues were homogenized in 1 ml of RIPA lysis buffer (25 mM Tris–HCl pH 7.4, 25 mM NaCl, 0.5 mM EDTA, 1% Triton–X–100, 0.1% SDS) for 30 min on ice and were centrifuged at 12000 x g for 10 min. For all supernatentsamples, a protease inhibitor cocktail was added (Roche, Mannheim, Germany) as well as 1 mM phenylmethanesulfonyl fluoride (PMSF) and phosphates inhibitors (1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride). Supernatants were then stored at –80oC until analysis. Upon thawing, protein content was assayed by the bicinchoninic acid method standardized to bovine erum albumin (Roche, Laval, QC, Canada) [13]. Lysates were diluted to a concentration of 1 mg⁄ml total protein and boiled for 5 min inreducing sample buffer (62.5 mMTris–HCl pH 6.8, 2% SDS, 10% glycerol, 5% ß–mercaptoethanol and 0.01% bromophenol blue). One hundred μL of each sample were separated on 10% polyacrylamidefull–size gels and transferred to nitro cellulose membrane (Millipore, Bedford, MA, USA). Membranes were blocked for 2 h at room temperature with Tween–20 and 5% skim milk in TBS (20 mM Tris– HCl, pH 7.6 and 137 mM NaCl). Membranes were then incubated overnight at 4°C in blocking buffer with appropriate phospho–specific or pan–specific antibodies against AMPK, ACC, GLUT4 (each at 1: 1000). Membranes were washed 5 times and incubated 1.5 h at roomtemperature in TBS plus Twine 20 with anti–rabbit HRP–conjugated secondary antibodies at 1:50000 to 1:100000 (Jackson Immune research, Cedar lane Laboratories, Horn by, ON, Canada). Revelation was performed using the enhanced chemiluminescence method and blue–light–sensitive film (Amersham Biosciences, Buckinghamshire, England). Gel band intensities were evaluated by densitometry analysis using Image Densitometry software (Version 1.6, National Institutes of Health and Bethesda, MD, USA).
Histological Analysis
Sections of excised livers were placed in the 10% formalin solution and were stained with hematoxylinphloxine saffron (HPS) by the Institute de Recherché en Immunologieet en Cancérologie (IRIC), Department of Histology (Université de Montréal, Montreal, QC, Canada). Each stained liver section was analyzed for the severity of lipid accumulation in the hepatocyte and was then scored based on the percentage of hepatocyte that contained macrovesicular fat: namely, grade 0 (0–5%), grade 1(5–33%), grade 2 (33–66%), and grade3 (66–100%), as previously described [14].
Statistical analysis
In vitro results as well as quantification of western blot data for in vivo studies were analyzed by one–way analysis of variance (ANOVA) using Stat View software (SAS Institute Inc, Cary, NC, USA), with post–hoc analysis as appropriate. Areas under the curve (AUC) were calculated by using PRISM software (Graph Pad, San Diego, CA, USA). Calculations of cumulative changes (body weight, food and liquid intake) were initiated several days prior to the onset of treatment in order to obtain baseline values. For the in vivo studies, Student’s t test for unpaired observations was used. Non–parametric data was analyzed by the Chi square test. Statistical significance was set at p≤0.05. Results are presented as the mean ± SEM for the indicated number of determinations or animals.
Results
V. vitis increases glucose uptake and GLUT4 translocation in L6 my tubes
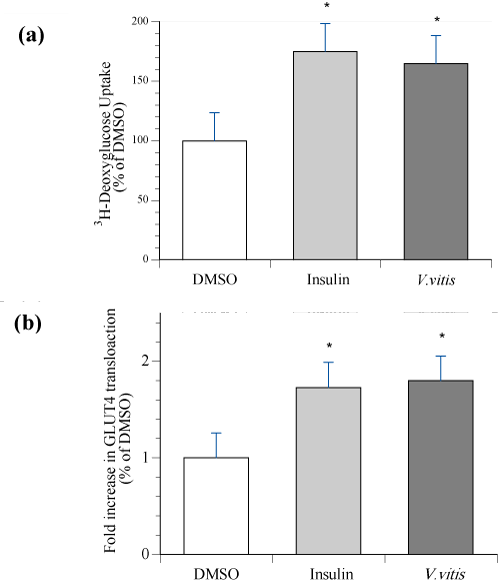
To confirm that V. vitis increases glucose uptake in L6–GLUT4mycmy tubes as it did in C2C12 cells [9], cells were treated with 200 μg⁄ ml V. vitis for 18 h or 100 nM insulin for 20 min and tested for [3H]– 2–deoxy–D–glucose uptake. V. vitis significantly stimulated glucose uptake by 65 ± 5 % above DMSO, p<0.05. This was comparable to the glucose uptake stimulated by insulin (positive control), whichreached 75 ± 13 % above DMSO (NS as compared to V. vitis; Figure 1a).
Using the OPD assay, we then examined the effect of V. vitis on GLUT4 translocation in L6–GLUT4mycmyoblasts. Our results showed that V. vitis significantly stimulated GLUT4 translocation in these cells with an efficacy very similar to that of insulin (both 1.8 fold increase relative to DMSO; Figure 1b, p<0.05).
Figure 1: V. vitis increased 3H–deoxyglucose uptake and GLUT4 translocation in L6 GLUT4myc myotubes. Cells were treated with either 200 μg⁄ml of V. vitis, or with vehicle (0.1% DMSO) for 18 h. 100 nM insulin was applied for the last 20 min of the treatment in vehicle–treated cells. (a) Glucose uptake. At the end of the treatment, cells were incubated with 3H–deoxyglucose then washed. Radioactivity was measured using a scintillation counter. Data are expressed after normalization to basal uptake observed in vehicle control treated cells. Data are presented as the mean of three experiments ± SEM, each experiment composed of 3–4 replicates per condition. (b) GLUT4 translocation. Cell surface GLUT4myc was detected by an enzyme–linked colorimetric assay. At the end of the treatment, L6– GLUT4myc myoblasts were labeled with anti–c–myc antibody as described in Materials and Methods section. The reaction of OPD was measured at 492 nm. Results represent the means ± SEM of three independent experiments, and 3–4 cells were analyzed for each condition per experiment. ∗ Indicates a significant difference (p ≤ 0.05) from the vehicle control group as assessed by ANOVA.
V. vitis increases the phosphorylation of AMPK in L6 my tubes
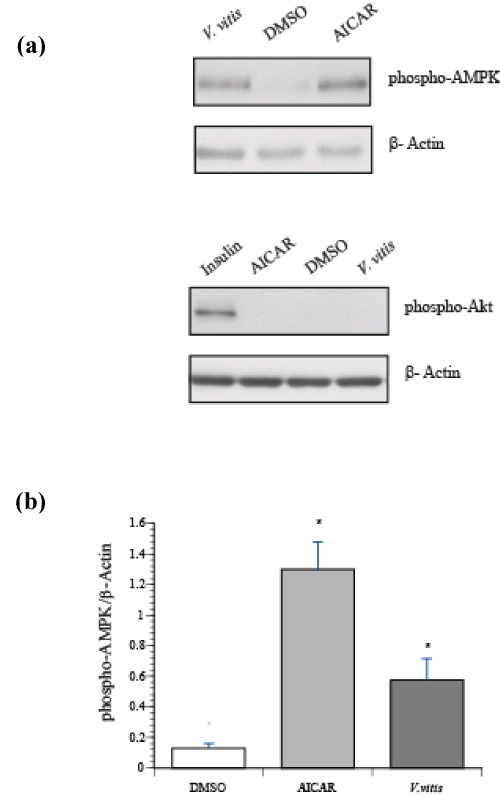
Two pathways control GLUT4 translocation in skeletal muscle: the insulin–dependent pathway through activation of PI3K⁄Akt and the insulin–independent pathway through activation of AMPK. Therefore, L6 my tubes were treated with either V. vitis extract (200 μg⁄ml; 18h), an activator of AMPK, AICAR (1 mM; 30 min) or insulin (100 nM; 20 min). Consistent with our previous observations in C2C12 myocytes [9], AMPK phosphorylation, and therefore its activation, was significantly increased by V. vitis treatment (Figure 2a, p<0.002). On the other hand, V. vitis treatment did not induce Akt phosphorylation, unlike insulin treatment (Figure 2b).
Figure 2: V. vitis increased phosphorylation of AMPK but not of Akt in L6 myotubes. Shown are representative immunoblots of cells treated with either vehicle (0.1% DMSO, 18 h), V. vitis (200 μg⁄ml, 18 h), AICAR (1mM, 30 min) or insulin (100 nM, 20 min). Immunoblots were probed with phospho–specific antibodies against (a) AMPK (Thr 172) and (b) Akt (Ser 473) as described in Materials and Methods section. Immunoblots were probed with β–actin as loading control. (c) Data are expressed 95 p–Ampk ⁄ β–actin and are given as mean ± SEM for three independent experiment.
V. vitis decreases body weight and glycemia in diabetic KKAy mice, but not in normal C57BL⁄6J mice
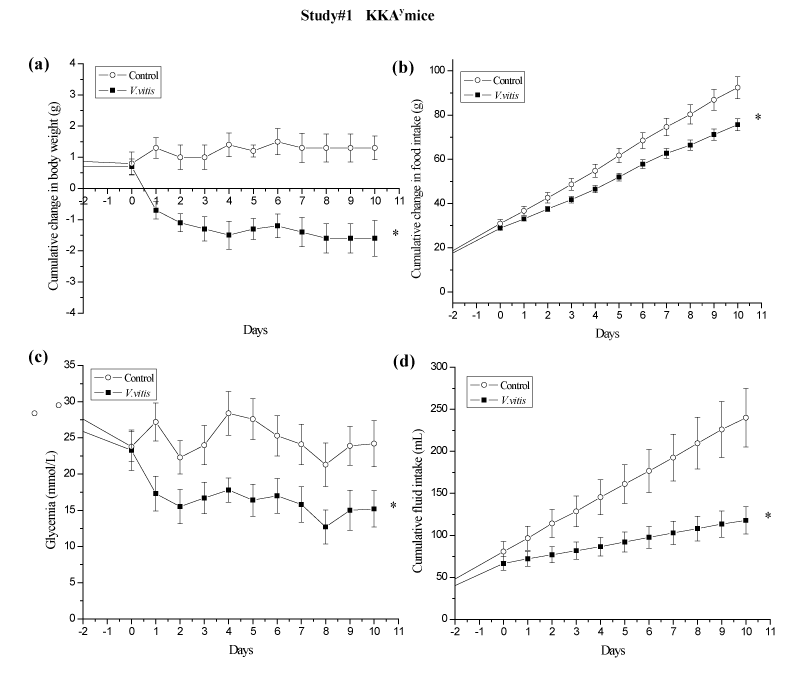
We next validated the anti–diabetic effect of V. vitis extract in an in vivo model, the KKAy mouse, where the plant extract was added to the drinking water (1%). In order to investigate the cumulative effect of V. vitis in time, we calculated AUC for each parameter measured. V. vitis treatment decreased body weight (BW) of diabetic KKAy mice in comparison to control mice receiving drinking water only. Indeed, V. vitis treated mice exhibited a cumulative drop in BW of 1.2 ± 0.4g, while control mice gained 1.1 ± 0.3g in the same period of time, corresponding to a reduction of AUC by 213% (p<0.05; Figure 3a). This was correlated with a significant reduction in AUC of cumulative food intake by 16% (p<0.05; Figure 3b) in V. vitis– treated group compared to vehicle control animals. Most importantly, daily administration of the plant extract to these mice resulted in a significant decrease of AUC of glycemia as compared to controls (32%; p<0.05; Figure 3c).
Figure 1: V. vitis exerts hypoglycemic and weight reduction effects in KKAy mice of study #1. During the 10 days of the study, cumulative changes in (a) body weight, (b) food intake, (c) non–fasting blood glucose concentration, and (d) fluid intake were recorded on a daily basis. ∗denotes significantly different as compared to control group (p < 0.05) as assessed by non–paired t test. N=7.
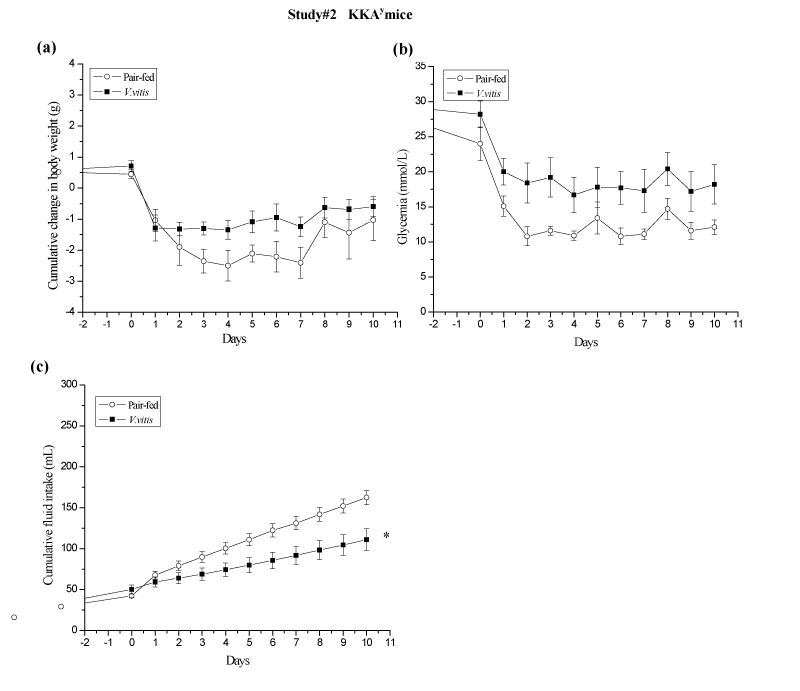
To test whether the reduction in food intake was responsible for the hypoglycemic and the weight reduction effects of V. vitis in these mice, study #2 was carried out where controls were pair–fed according to the food intake of the V. vitis–treated group (Figure 4). Both pairfed and V. vitis–treated mice had a similar loss in body weight, albeit pair–fed animals exhibited a slight tendency for greater cumulative weight loss (–1.0 ± 0.7 g vs. –0.5 ± 0.3 g respectively; N.S.; Figure 4a). In addition, V. vitis administration and pair feeding significantly reduced AUC of glycemia by a similar extent when compared to theirrespective baseline levels (42% and 56% respectively, <0.05; Figure 4b). This suggests that food intake, body weight and glycemia are closely associated in V. vitis treated animals.
Figure 4: V. vitis–treatment and pair–feeding reduced body weight and glycemia in KKAy mice of study #2. (a) Cumulative body weight, (b) non–fasting blood glucose concentration and (c) fluid intake were recorded every day for 10 days. ∗ denotes significantly different as compared to pair–fed mice (p ∗ 0.05) as assessed by non paired t test. N = 7.
Finally, V. vitis was administered to normal C57BL⁄6J mice to study its effects in non–diabetic animals (study #3; Figure 5). In contrast to KKAy studies, no significant changes in glycemia were observed in V. vitis–treated animals as compared to controls (N.S.; Figure 5c). Interestingly, normal mice treated with V. vitis exhibited a modest tendency to lose weight as compared to control animals (–0.5 ± 0.1 g vs +0.1 ± 0.1 g respectively; N.S.; Figure 5a). This occurred despite the fact that a slight, albeit significant, increase in AUC of cumulative food intake was observed during the same time period for V. vitis–treated animals (10.5%, p<0.05 compared to water control animals; Figure 5b).
Noteworthy, AUC of cumulative fluid intake was significantly reduced by V. vitis treatment in all three studies as compared to their respective controls: by 44 %, 31 % and 22 % instudies #1 (p<0.05; Figure 3d), #2 (p<0.05; Figure 4c) and # 3 (p<0.05; Figure 5d), respectively.
Figure 5: V. vitis had no effect on body weight or glycemia in normal C57BL⁄6 mice of study #3. Cumulative changes in (a) body weight, (b) food intake, (c) non–fasting blood glucose concentration and (d) fluid intake were recorded for 10 days every day. All values are mean ± SEM. ∗denotes significantly different as compared to control mice (p < 0.05) as assessed by unpaired t test. N=7.
Effect of V. vitis onhepatic steatosis and insulin resistance in KKAy mice
We evaluated hepatic steatosis by assessing lipid accumulation in liver tissue sections according to histological scoring. In control KKAy diabetic mice of study #1, 29% (2 out of 7 mice) had grade 0 or 1 steatosis, while 71% had grade 3 steatosis. V. vitis treatment increased the proportion of mice exhibiting weak or no steatosis (grades 0 or 1) to 57 % while concomitantly reducing to 43% the number of animals with grade 3 steatosis (N.S, Table 1). In study #2, pair–feeding itself yielded a better steatosis profile than the control animals in the first study. Indeed, 71% of animals were essentially free of steatosis (grade 0), while 28% had moderate to severe steatosis (grade 2 or 3). Interestingly, the effects of V. vitis were more pronounced in study #2 since no grade 2 or 3 were recorded. All seven animals were either grade 0 or 1, yet this result was not statistically different from that of pair–fed congeners (N.S, Table 1).
Grade of Steatosis
Study #1
Groups
n
0
1
2
3
Control
7
1
1
0
5
V .vitis
7
3
1
0
3
Study #2
Pair-fed
7
5
0
1
1
V .vitis
7
4
3
0
0
Table 1: Histological scores of liver steatosis from KKAy diabetic mice of study #1 and study #2.
Administration of V. vitis to KKAy mice in study #1 reduced plasma insulin and triglyceride levels by 46% and 36 % respectively as compared to their respective controls, although this failed to reach statistical significance possibly due to data variability (Table 2). Other blood lipid parameters including total cholesterol, HDL–C and LDL–C, as well as plasma leptin and adiponectin levels, and leptin⁄ adiponectin ratio were not significantly affected by plant treatment (Table 2). Moreover, V. vitis treatment did not significantly affect parameters of liver or kidney function (data not shown). In addition, epididymal fat, abdominal fat and dorsal fat pads did not show any significant differences in weight (not illustrated).
Study #1
Study #2
Control
V. vitis
Pair-fed
V. vitis
Triglycerides (mmol/L)
4.5 ± 0.8
2.9 ± 0.5
2.9 ± 0.6
2.7 ± 0.5
Cholesterol (mmol/L)
2.3 ± 0.1
2.1 ± 0.2
3.2 ± 0.4
2.3 ± 0.3
HDL (mmol/L)
1.1 ± 0.1
1.1 ± 0.1
1.4 ± 0.2
1.0 ± 0.1
LDL (mmol/L)
0.5 ± 0.2
0.5 ± 0.0
0.5 ± 0.0
0.5 ± 0.2
Insulin (ng /ml)
35.3 ± 8.4
19.0 ± 4.2
6.0 ± 2.1
12.8 ± 2.1*
Leptin (ng /ml)
27.1 ± 1.7
23.9 ± 0.9
29.1 ± 1.6
27.3 ± 3.0
Adiponectin (μg /ml)
18.2 ± 2.4
18.0 ± 2.4
12.6 ± 1.5
11.4 ± 2.0
Leptin / adiponectin
1.6 ± 0.2
1.4 ± 0.2
2.4 ± 0.4
3.0 ± 0.5
Table 2: Blood parameters of KKAy mice from study #1 and study #2.
Effect of V. vitis on GLUT4 total protein content in skeletal muscle of KKAy mice
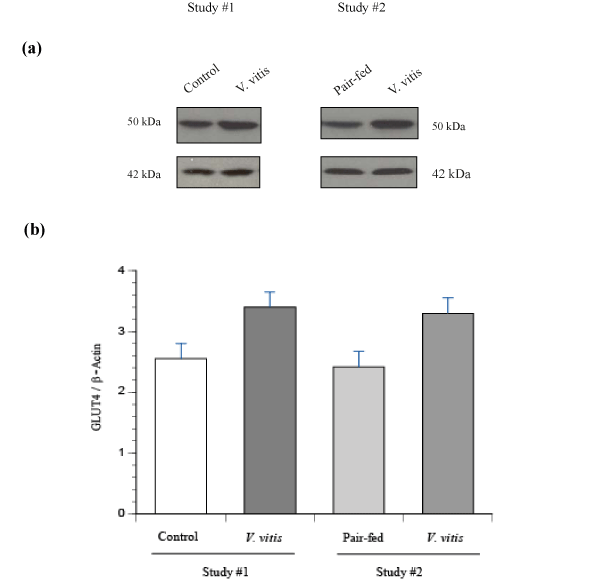
Since V. vitis stimulated GLUT4 translocation and glucose uptake in muscle cells in culture, we were interested in the effect of the plant on GLUT4 protein content in skeletal muscle (soleus muscle) tissues of KKAy mice harvested at the end of studies #1 and #2. GLUT4 protein levels exhibited a strong and consistent tendency to increase with V. vitis treatment, whether it was in study #1 (N.S, p=0.18; Figure 6a & 6b) or in the pair–feeding study #2 (N.S, p=0.06; Figure 6a & 6b).
Figure 6: Effect of V. vitis treatment on GLUT4 content in soleus muscle of diabetic KKAy mice of study #1 and study #2.Samples of soleus muscles were obtained from control, V. vitis and pair–fed diabetic KKAy mice and analysed by immunoblotting with antibodies specific to GLUT4. (a) Representative blots from study #1 and #2 are shown. (b) Data are expressed as GLUT4⁄β– actin and is given as mean ± SEM from 7 animals.
Since V. vitis activated AMPK in L6 my tubes in vitro, we investigated the involvement of this pathway in vivo as well. We therefore compared phosphorylation levels of ACC, a stable downstream substrate of AMPK, in the muscle of control, pair–fed and V. vitis treated KKAy mice from the first two studies. Our results showed that there was no significant difference in the phosphorylation levels of ACC between control, pair–fed and V. vitis–treated mice(data not shown).
Discussion
Aboriginal populations around the world are particularly at risk of developing metabolic disorders related to lifestyle changes. When risk factors like obesity and genetic predisposition entwine with the cultural disconnection of modern medication, the complications of diabetes also increase and can become debilitating and life threatening [5,15,16]. To address this problem in Canadian aboriginal populations, our team has explored potential anti–diabetic plants that could represent promising and culturally relevant alternative and complementary treatment options for managing diabetes in these populations. In collaboration with the Cree Elders and Healers ofEeyouIstchee (James Bay area of QC, Canada), we have identified the extract of Vaccinium vitis–idaea L. berries as a promising productcapable of enhancing glucose uptake in skeletal muscle cells in culture [8].
V. vitis is a rich source of polyphenols including flavonoids, phenol acids, tannins, proanthocyanidins and stilbenes [17–19]. The most common Flaronoids in V. vitis are the flaronols quercetin and kaempherols, together with their glycosides but querectin glycosides are the most abundant. Other important Flaronoid classes such as catechins are also detected in V. vitis. The content of catechins is 10 times higher in V. vitis than in the other Vaccinium species[20]. A bioassay guided fractionation studies done by our team on V. vitis berries ethanolic extract identified quercetin and quercetin glycosides as the active principles responsible for the enhancement of glucose uptake in skeletal muscle cells. In total, 12 compounds including the active compounds were identified by high performance liquid chromatography ⁄ mass spectrometry analysis. The major compounds were benzoic acid, p–coumaric acid, cyandin glucoside, cyanidin galactoside and p–coumaroyl–D–glucoside [9].
Skeletal muscle is the major site of glucose disposal accounting for 80% of insulin–stimulated glucose up take [21]. Skeletal muscle cells express two is of forms of facilitative glucose transporters, GLUT4and GLUT1. The latter resides mainly in the plasma membrane and is responsible for basal glucose transport. On the other hand, GLUT4 resides in intracellular vesicles and translocates to the plasma membrane in response to insulin stimulation [22]. In accordance with previous observations [8,9], V. vitis significantly increased cellular glucose uptake. The effect of V. vitis on glucose uptake in L6 cells (65% increase) was more than double that observed previously in C2C12 myotubes (31%; [9]). This was not unexpected and can likely be attributed to the higher content of GLUT4 in L6 cells [23].
V. vitis treatment also leads to the activation of the insulin independent AMPK pathway but not of insulin–dependent Akt in L6 my tubes. This is in accordance with previous studies implicating the AMPK pathway and mitochondrial inhibition as the mechanism ofaction of V. vitis and its active principles in C2C12 my tubes [9]. In the present study, we confirmed that the V. vitis–induced increase in glucose uptake was associated with increased translocation of GLUT4to the plasma membrane using L6 cells expressing GLUT4myc. Most importantly, the extent of glucose transport stimulation and GLUT4 translocation induced by V. vitis administration was equivalent to that of an optimal dose of insulin in these cells. This reinforces the promising antidiabetic potential of V. vitis extract raised in our recent in vitro studies [9]. This is also in accordance with previous in vivo reports on the anti–diabetic activity of the plant’s active principles:quercetin and quercetin–3–O–glucoside [24–26].
In view of such precedents, it seemed fitting to investigate theanti–diabetic effect of V. vitis in vivo; therefore, we selected the aforementioned diabetic KKAy mouse model. Unexpectedly, V. vitistreatment reduced water intake in all three in vivo study protocols, albeit with varying intensity. Indeed, there was a 44% drop in cumulative fluid intake when V. vitis was administered to KKAy mice as compared to their cognate controls (study #1). This difference was diminished to 31% in the pair–feeding study (study #2), suggesting that part of the V. vitis effect on fluid intake can be related to the expected decrease in blood tonicity and polyuria that results fromthe reduction in food intake (pair–feeding “forcing” this on control animals as well) and associated decrease of glycemia. The remaining component in the decrease of water intake by this plant could be explained, at least in part, by the acidic and astringent properties of V. vitis berries; traits shared with the plant’s cousin, cranberries, and potentially representing unpleasant organoleptic features for mice. Indeed, even normal C57BL⁄6J mice in study #3 exhibited a significant, albeit more modest, decrease in fluid intake (22% as compared to the first 2 studies). This appears sensible, given that normal mice do not have the added strain of hyperglycemia (with expected blood hypertonicity and polyuria) of their KKAy cousins that would be consistent with higher fluid intake in the latter. Taste aversion tests will be necessary to confirm this interpretation.
More importantly, V. vitis extract administration exerted a rapid, considerable and persistent effect to significantly lower cumulative changes in body weight, food intake and glycemia. These results suggest that the plant may possess weight reducing, antihyperglycemic and appetite–modifying effects. This led us to carry out study #2, where the use of pair feeding demonstrated that the blood glucose lowering and weight reducing effects of V. vitis could be attributed in major part to reduced food intake.
Surprisingly, despite a fully comparable pattern of weight changes, pair–fed animals exhibited significantly lower levels of blood glucose compared to V. vitis treated animal. One possible explanation for this counter–intuitive result stems from the significant sugar content of V. vitis berries that can reach up to 8% of their fresh weight [27] and 27% of their dry weight. Although we did not measure the exact amount of sugar consumed by our animals, he sugar provided by V. vitis administration nonetheless enhances the significance of the anti–hyperglycemic and weight–reducing actions observed with plantextract treatment in diabetic KKAy. Notwithstanding, our results do indicate that the antihyperglycemic effect of V. vitis is mediated in good part by the reduction of food intake. The underlying mechanisms remain to be fully elucidated, but they do not appear to involve the satiety hormone leptin whose circulating levels were not affected by V. vitis. Further investigations, notably concerning leptin signaling,will be needed to clarify this point. However, we found no evidence that the reduced food intake was related to any toxic action of the plant. Indeed, we did not observe any behavioral or external (e.g. fur, eyes) changes indicating toxicity (not illustrated). Finally, the plant’s berries have been and continue to be consumed by the CEI and other populations, thereby confirming their GRAS (generally regarded as safe) status.
In contrast with KKAy mice, the administration of V. vitis to normal C57Bl/6J animals did not affect body weight or blood glucose levels. A possible explanation for this and the food intake reduction inKKAy mice might stem from the disruption of central melanocortin (MC) system in the latter. This system regulates feeding behavior and energy expenditure and its disruption is responsible for the KKAy phenotype of hyperphagia, hyperlipidemia, hyperleptinemia, maturity–onset obesity and diabetes [28]. Since antagonism of this system has been shown to control body weight [29,30], it is tempting to propose that V. vitis could act similarly in KKAy mice. This brings forth its potential therapeutic utility in humans with genetic defects in the MC system. Further studies specifically aimed at the MC system will be required to address these issues.
However, this hypothesis cannot account for the fact that normal lean V.vitis–treated C57BL⁄6L mice had a slight but significant increase in food intake in contrast to the opposite effect in KKAy mice. These counter intuitive results may stem from our previous observation that V. vitis acts as a mild mitochondrial inhibitor [9]. Indeed, such disruption of mitochondrial energy transduction (also caused by induction of uncoupling proteins – UCPs) is reminiscent of the stimulatory effects of chronic moderate to intense physical activity on mitochondrial biogenesis by inducing AMPK activation [31]. Moreover, when lean animals and humans carry out regular moderate to vigorous exercise, a negative energy balance is created. This triggers a compensatory increase in food intake that does not affect body mass [32–34], similar to what we observed in C57BL/6L mice consuming V. vitis extract. By virtue of its mitochondrial inhibitory and AMPK enhancing actions, V. vitis could thus create a negative energy balance by increasing energy expenditure, particularly in normal mice, andfuture studies should address this point.
This interpretation is fully compatible with the insights into the cellular and molecular mechanisms underlying the antihyperglycemic action of V. vitis that were obtained from our in vitro results in both C2C12 [9] and L6 muscle cells (presented above). These showed that V. vitis increased glucose transport and GLUT4 translocation to themembrane, associated with an activation of the AMPK pathway. This pathway has been shown to increase synthesis of GLUT4 in skeletal muscle [35], thus resulting in enhanced glucose transport capacity of this organ. We failed to observe the enhancement of the AMPK pathway in skeletal muscle of V. vitis treated group. This could be due to the fact that harvesting the organs at sacrifice represents a snapshotin time whereby dynamic changes in the phosphorylation of the AMPK pathway components (such as ACC in the present studies) may not be detected consistently. However, V. vitis treatment exhibited a strong tendency to increase muscle GLUT4 content in diabetic KKAy mice, which could have contributed to the concomitant decrease in glycemiain these animals. However, the total level of GLUT4 protein in skeletal muscle may not provide a definitive indication of the ability of the tissue to uptake glucose.
Concomitantly, a number of parameters indicate that V. vitis can also improve insulin sensitivity. Firstly, we found that plant extract–fed mice had decreased plasma insulin levels. In addition, V. vitis intake tended to reduce liver steatosis in these diabetic mice. Indeed, hepatic steatosis is closely associated with obesity and insulin resistance, leading to exaggerated hepatic glucose production [36]. Despite some heterogeneity in the effects of V. vitis between studies#1 and #2, results were nonetheless consistent since subgroups responding better to V. vitis in terms of steatosis were also the onesexhibiting lower insulin levels. Nonetheless, our results highlight the potential of V. vitis berry extract to exert pleiotropic effects to increase hepatic insulin sensitivity by reducing liver steatosis.
Conclusions
In summary, the results of the present study clearly demonstrate that V. vitis possesses significant anti–diabetic and anti–obesity actions. These are exerted through a combination of pleiotropic effects on different organs including reduction of food intake, increased glucose transport in skeletal muscle and reduced hepatic steatosis. Our studies thus pave the way toward clinical assessments of V. vitis products, notably in the context of a culturally relevant approach to diabetes care in the CEI.
Acknowledgements
Special thanks are given to Cree Elders of EeyouIstchee who kindly agreed to share their traditional knowledge relating to medicinal plants: Elizabeth Coon Come, Mable Gunner, Charlotte Husky Swallow, Johnny Husky Swallow, Ronny Loon and Girty Loon from the Cree Nation of Mistissini; Eliza Kawapit, Abraham Mamianskum, Andrew Natachequan, Maggie Natachequan and John Petagumskum from Whapmagoostui First Nation; as well as 54 other Cree Elders and healers from either nation. They made this article possible by allowing us to use, for the purposes of this research, their knowledge relating to medicinal plants transmitted to them by their Elders. Their trust has also enabled a useful exchange between Indigenous knowledge and Western science.
References
- Helmstädter A, Schuster N. Vaccinium myrtillus as an antidiabetic medicinal plant--research through the ages. Pharmazie. 2010; 65: 315-321.
- Wilson T, Singh AP, Vorsa N, Goettl CD, Kittleson KM. Human glycemic response and phenolic content of unsweetened cranberry juice. J Med Food. 2008; 11: 46-54.
- Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006; 13: 612-623.
- Vuong T, Matar C, Ramassamy C, Haddad PS. Biotransformed blueberry juice protects neurons from hydrogen peroxide-induced oxidative stress and mitogen-activated protein kinase pathway alterations. The British journal of nutrition. 2010; 104: 656-663.
- Young TK, Reading J, Elias B, O'Neil JD. Type 2 diabetes mellitus in Canada's first nations: status of an epidemic in progress. CMAJ. 2000; 163: 561-566.
- Fraser MH, Cuerrier A, Haddad PS, Arnason JT, Owen PL. Medicinal plants of Cree communities (Québec, Canada): antioxidant activity of plants used to treat type 2 diabetes symptoms. Can J Physiol Pharmacol. 2007; 85: 1200-1214.
- Leduc C, Coonishish J, Haddad P, Cuerrier A. Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: A novel approach in quantitative ethnobotany. J Ethnopharmacol. 2006; 105: 55-63.
- Harbilas D, Martineau LC, Harris CS, Adeyiwola-Spoor DC, Saleem A. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: part II. Can J Physiol Pharmacol. 2009; 87: 479-492.
- Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, et al. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res. 2010; 54: 991-1003.
- Adachi Y, Yoshikawa Y, Yoshida J, Kodera Y, Katoh A. Improvement of diabetes, obesity and hypertension in type 2 diabetic KKAy mice by bis(allixinato)oxovanadium(IV) complex. Biochem Biophys Res Commun. 2006; 345: 945-950.
- Niu W, Huang C, Nawaz Z, Levy M, Somwar R. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003; 278: 17953-17962.
- Cignarella A, Nastasi M, Cavalli E, Puglisi L. Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic treatment, in several models of rat dyslipidaemia: a comparison with ciprofibrate. Thromb Res. 1996; 84: 311-322.
- Simpson RJ. Quantifying protein by bicinchoninic Acid. CSH Protoc. 2008; 2008: pdb.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999; 94: 2467-2474.
- Brassard P, Robinson E, Lavallée C. Prevalence of diabetes mellitus among the James Bay Cree of northern Quebec. CMAJ. 1993; 149: 303-307.
- Hegele RA. Genes, environment and diabetes in Canadian aboriginal communities. Adv Exp Med Biol. 2001; 498: 11-20.
- Wang SY, Feng R, Bowman L, Penhallegon R, Ding M, et al. Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-kappaB, and mitogen-activated protein kinases activation. J Agric Food Chem. 2005; 53: 3156-3166.
- Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004; 52: 4713-4719.
- Kähkönen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001; 49: 4076-4082.
- Määttä-Riihinen KR, Kamal-Eldin A, Törrönen AR. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J Agric Food Chem. 2004; 52: 6178-6187.
- Ryder JW, Chibalin AV, Zierath JR. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand. 2001; 171: 249-257.
- Mitsumoto Y, Burdett E, Grant A, Klip A. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun. 1991; 175: 652-659.
- Mitsumoto Y, Klip A. Development regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem. 1992; 267: 4957-4962.
- Kannappan S, Anuradha CV. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J Med Res. 2009; 129: 401-408.
- Panda S, Kar A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors. 2007; 31: 201-210.
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003; 135C: 357-364.
- Hjalmarsson I, Ortiz R (2010) Lingonberry: Botany and Horticulture. In: Horticultural Reviews (Janick J, ed.), Oxford: John Wiley & Sons, Inc.
- Nonogaki K, Nozue K, Oka Y. Hyperphagia alters expression of hypothalamic 5-HT2C and 5-HT1B receptor genes and plasma des-acyl ghrelin levels in Ay mice. Endocrinology. 2006; 147: 5893-5900.
- Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, et al. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes. 2000; 49: 244-252.
- Ollmann MM, Barsh GS. Down-regulation of melanocortin receptor signaling mediated by the amino terminus of Agouti protein in Xenopus melanophores. J Biol Chem. 1999; 274: 15837-15846.
- Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003; 551: 169-178.
- Melzer K, Kayser B, Saris WH, Pichard C. Effects of physical activity on food intake. Clin Nutr. 2005; 24: 885-895.
- Woo R, Garrow JS, Pi-Sunyer FX. Voluntary food intake during prolonged exercise in obese women. Am J Clin Nutr. 1982; 36: 478-484.
- Woo R, Garrow JS, Pi-Sunyer FX. Effect of exercise on spontaneous calorie intake in obesity. Am J Clin Nutr. 1982; 36: 470-477.
- Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004; 63: 275-278.
- Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002; 87: 3023-3028.