
Research Article
Austin J Endocrinol Diabetes. 2016; 3(2): 1042.
Berberine, Quercetin and O-Coumaric Acid Phytochemicals Ameliorate the Impact of Experimentally Fed High-Fat/High-Sucrose Diet on Pancreas Β-Cells and Glycemic Control Indices
Hossam El-Din M Omar1*, Sohair MM Ragaa1, Sary Kh Abd Elghaffar2, Abdulrahman A Alduraywish3 and Tarek H El-Metwally4
1Department of Zoology, Faculty of Sciences, Assiut University, Egypt
2Department of Pathology and Clinical Pathology, Faculty of Vet. Medicine, Assiut University, Egypt
3Department of Internal Medicine, College of Medicine, Aljouf University, Saudi Arabia
4Department of Medical Biochemistry, College of Medicine, Aljouf University, Saudi Arabia, and, Assiut University, Egypt
*Corresponding author: Hossam El-Din M Omar, Physiology Lab, Department of Zoology, Faculty of Science, Assiut University, Egypt
Received: May 18, 2016; Accepted: June 15, 2016; Published: June 20, 2016
Abstract
Background: The worldwide growing pandemic of diabetes necessitates much attention to combat the pathogenetic metabolic syndrome.
Aim: The present study aimed at investigating the possible ameliorative effect of oral administration of each of Quercetin (Q), O-Coumaric Acid (CA) and Berberine (BB) natural phytochemicals on pancreatic β-cells and glycemic control impact of High Fats/High Sucrose (HFS) diet in Wistar Albino rats.
Methods: Fifty young adult animals (100-120 g body weight) were classified into 5 groups; normal diet-fed control group, HFS diet-fed control group, and, 3 HFS diet-fed treatment groups. After 6 weeks of induction, each of HFS diet-fed treatment groups was treated with Q, CA, or BB for a further 6 weeks. Light and EM histopathological changes, morphometric pancreatic islet mass and glycemic control indices (fasting serum glucose and insulin levels and insulin resistance) were evaluated.
Results: Rats fed HFS diet suffered from hyperglycemia and hyperinsulinemia that both caused insulin resistance accompanied with increase in islet mass and degeneration of β-cell granules. Treatment with Q, CA or BB reversed the biochemical and histological changes, albeit, BB was the most efficient. Conclusion: The investigated phytochemicals restored glycemic control and insulin sensitivity in rats fed HFS diet with BB as the strongest β-cells pancreato-protective.
Keywords: Phytochemicals; High fat/high sucrose diet; Insulin resistance; Pancreas; β-cells; Quercetin; O-Coumaric acid; Berberine
Introduction
Generations born after the year 2000 have tripled obesity prevalence compared to those of the 1980s. This is reasoned to their physical inactivity and obesogenic diet and contaminants [1]. Pathogenesis of metabolic syndrome implicates metabolic dysregulation of lipid and glucose along with islet and/or insulin dysfunction [2]. Accumulated adipose tissue and subsequent changes in cytokines/fatty acids pattern affect glucose uptake, lipid metabolism, inflammation, and vascular homeostasis. This is due to peripheral Insulin Resistance (IR) in insulin-dependent tissues (liver, adipose tissue and skeletal muscle) [3,4]. Clinical and preclinical studies indicate that body lipids loss/gain correlates closely with increasing/decreasing insulin sensitivity [5,6]. Hyperinsulinemia ensues because of the adaptative compensatory pancreatic β-cells hyper function to overcome peripheral IR [7-9]. On the long term, however, β-cells failure ensues [10], leading to the development of type 2 diabetes [11]. High fat and high sucrose foods are the main elicitors of the metabolic syndrome complex [12,13]. Long-term administration of diets containing 40 - 60% lipids promotes the induction of obesity and IR experimentally and in human [14], and induces adipocyte hypertrophy [15] and hypertriglyceridemia [16].
Complementary and alternative medicine possess broad spectrum arena of anti-diabetic therapeutics [17]. Phytochemicals - not yet classified as essential nutrients, have health-promoting properties [18]. They include phenolic compounds such as Quercetin (Q) and utilize multiple mechanisms to combat the hyperglycemia-related diseases [19,20]. Fruits, vegetables, grains, tea, coffee, and spices consumed daily are rich in such phytochemical phenolics [21,22]. Alkaloids such as Berberine (BB) as another class of phytochemicals potently reduce body weight, improve glucose tolerance and insulin action in obese and/or diabetic subjects [23,24].
Antiobesogenic antioxidant phenolics, e.g., o-Coumaric Acid (CA) block various stages of adipocyte development culminating into its apoptosis, and stimulate lipolysis while inhibiting lipogenesis – as reflected on reduced adipose tissues mass and body weight. For that, they reduce the expression of each of glycerol-3-phosphate dehydrogenase, PPARγ, C/EBPα and leptin while up-regulating the expression of adiponectin at the protein and mRNA levels [25-29]. The present study aimed at investigating the potential ability of oral administration of each of Q, CA or BB against high-fat/high-sucrose- induced metabolic syndrome/type 2 diabetes in Wister Albino rats utilizing histopathological changes, islet mass and glycemic control indices (fasting serum glucose and insulin, and insulin resistance) as end-point biomarkers.
Material and Methods
Animals
Fifty young adult Wistar rats (six-week old 100-120 g body weight) were purchased from the Animal House, Faculty of Medicine, Assiut University, Assiut, Egypt. They were housed and acclimatized for experimentation at Zoology Department. All of the animal procedures were performed in accordance with the guidelines for the care and use of experimental animals established by the Committee for the Purpose of Control and Supervision of Experiments on Animals. In a well-ventilated room and in metal cages, animals were maintained under standard laboratory conditions (25 - 30 °C, 60 - 70% relative humidity and a 12-hour light/dark cycle).
Experimentation
Rats were randomly divided into 2 main groups: a normal diet control group of 10 rats that were fed a standard diet ad libitum (SD; 80% carbohydrates, 18% proteins and 2% fats) and 40 rats that were fed High Fat-High Sucrose (HFS) diet (55% SD diet, 15% beef tallow, 10% sucrose, 5% roasted peanuts, 5% milk powder, 5% whole eggs, 3% sesame oil and 2% NaCl) plus 10% sucrose in their drinking water; both ad libitum. After 6 weeks of induction, these 40 rats were subdivided into four groups. The first HFS diet control was left to continue untreated on HFS for the further 6 weeks. Each of the other 3 groups was treated with daily gavage of Q (50 mg/kg b.w.), CA (75 mg/kg b.w.) or BB (50 mg/kg b.w.) for the further 6 weeks on top of the continuing HFS diet. Q and CA were dissolved in 10% DMSO and BB was dissolved in warm saline solution. The 3 compounds and solvent were purchased from Sigma-Aldrich Co. (St Louias, MO, USA).
Sampling and biochemical investigations
Overnight fasting animals were bled from jugular vein under light diethyl ether anesthesia to recover morning (from 8 to 9 am) serum after clotting and centrifugation at 6,000 rpm for 10 minutes at 4°C. Sera were aliquot stored at -80 °C. Rats in the different groups were then killed by cervical dislocation. The pancreas was quickly removed and fixed in 10% neutral buffered formalin for the histopathological investigations. Serum glucose was determined enzymatically using commercially available reagent kit (Egyptian Company for Biotechnology, SAE, Cairo, Egypt). Insulin was measured by sandwich DRG insulin ELISA kit (EIA-2943, DRG International, Inc., Springfield, New Jersey 07081, USA - with lower detection limit of ≤0.020 μg/L = 0.46 mU/L = 2.76 pM/L). Insulin Resistance (IR) was calculated using the Homeostatic Model Assessment (HOMA) assuming normal insulin resistance of ≤1 [30]. HOMA-IR = Fasting insulin (μU/mL) X Fasting glucose (mM/L) / 22.5. Literatures show its applicability to rats with possible advantages over tolerance tests [31-35].
Histopathological examination and electron microscopic study
Parts of pancreatic tissues fixed in 10% neutral buffered formalin were processed according to standard procedures. Sections (7 μm) of the different groups were mounted on slides and dried overnight at 37 °C. The sections were de-waxed in xylene, hydrated in a graded series of alcohol solutions and then stained with hematoxylin and eosin for histological evaluation. Other small pancreatic tissue fragments were cut into 1-mm3 sections, immediately fixed in 2.5% glutaraldehyde and rinsed in 0.1 M phosphate buffer. After fixation in 1% osmium tetroxide and rinsing in 0.1 M phosphate buffer, the samples were dehydrated in a graded series of alcohol solutions and embedded in pure epoxy resin. Ultrathin sections (50-80 nm) were cut with a Leica AG Ultra microtome and stained with uranyl acetate and lead citrate. The sections were examined with a TEM (Jeol, 100 CXII) operated at 80 KV at the Electron Microscopic Center, Assiut University. The mean diameter of pancreatic islets was measured by histopathological examination of 3 sections from different parts of the pancreas for each animal. Semi quantitative morph metric analysis of the diameter of the islet mass was expressed as % of normal control and was done by research microscope (Carl Zeiss Axiovision Product SDVD 30) as we had previously described [36].
Statistical analysis
The data were tested for normality using the Anderson-Darling test and for homogeneity of variances prior to further statistical analyses. The data were normally distributed and were expressed as the mean ± Standard Error of the Mean (SEM). The significant of differences among groups was analyzed using a one-way ANOVA followed by a Newman-Keuls multiple comparisons test using PRISM 6 Statistical Software (GraphPad Software Inc., San Diego, CA, USA). Differences were considered statistically significant at p<0.05.
Results
Changes in serum glucose and insulin levels as reflected on insulin resistance
After 12 weeks on HFS diet, there were significant increases in serum glucose (mg/dL; p<0.001) in HFS fed rats as compared to normal control rats. Treatment with each of Q, CA or BB for the subsequent six weeks (after 6 weeks of induction on HFS diet) significantly reduced serum glucose level (p<0.001) as compared to HFS-fed control rats to a level non-significantly different from normal diet controls. There were non-significant differences among the 3 phytochemicals in normalizing serum glucose (Table 1). Serum insulin level (μU/mL) of HFS-fed rats increased significantly (p<0.05) in comparison with normal diet controls. Treatment with each of Q, CA, or BB caused significant decrease in serum insulin level as compared to HFS-fed control rats (p<0.05) without significant differences amongst them or comparing each of them vs. normal diet rats (Table 1). Insulin resistance calculated as HOMA-IR showed normalization of the HFS diet-induced insulin resistance to ≤1, the normal upper limit, following treatment with each of Q, CA and BB (p<0.001; Table 1). There were non-significant differences amongst the three treatments and comparing each of them vs. normal diet rats. Morphometric analysis showed that HFS-fed rats and HFS-fed rats treated with either Q or CA had significant increase in the mean diameter of pancreatic islets compared to normal diet rats (p<0.001, 0.05, and 0.05, respectively) without significant difference among the three of them. Comparing HFS-fed rats treated with BB vs. normal diet rats there was non-significant difference although BB treatment was also non-significantly different from HFS-fed rats and those treated with Q or CA (Table 1).
Parameters
NC
HFS
HFS+Q
HFS+CA
HFS+BB
Glucose, mg/dL
136.1 ± 2.39
191.5 ± 9.14a***
149.6 ± 10.21b***
145.5 ± 4.48b***
150 ± 6.99b***
Insulin, µU/mL
1.59 ± 0.147
1.74 ± 0.317a*
1.51 ± 0.73b*
1.28 ± 0.202b*
1.36 ± 0.052b*
HOMA-IR
0.634 ± 0.04
1.171 ± 0.122a***
0.657 ± 0.062 b***
0.628 ± 0.053 b***
0.532 ± 0.033b***
MIMD, μm
92 ± 14
232 ± 23a**
215 ± 29
205 ± 33
153 ± 32
HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; a: significant difference comparing Normal Controls (NC) vs. HFS groups; b: significant difference comparing HFS vs. each of Q, CA or BB-treated groups; * = p<0.05, and, *** = p<0.001.
Table 1: Effect of oral administration of each of Quercetin (Q), o-Coumaric Acid (CA) or Berberine (BB) for 6 weeks on glycemic control indices (morning fasting serum glucose and insulin levels and insulin resistance) and Mean Islet Mass Diameter (MIMD) of High-Fat/High Sucrose (HFS) fed Wister Albino rats. Data shown are mean ± SEM, where n = 8 for each group.
Light and electron microscopic changes in pancreatic islets
Staining with H&E revealed that the pancreas of normal diet rats showed the normal pancreatic acini and pancreatic islets (Figure 1A). HFS fed rats showed marked enlargement and hyperplasia of pancreatic islets that was slightly reduced following treatment with each of Q, CA and BB (Figure 1B/C, D, E and F, respectively).
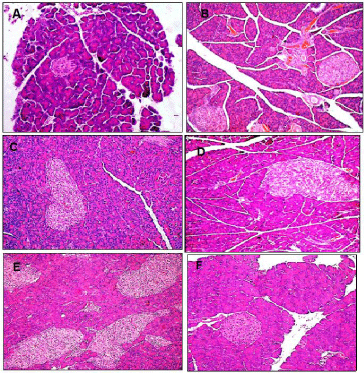
Figure 1: Representative light microscopic H&E micrographs showing pancreatic islets of healthy normal diet control (A), High Fat-High Sucrose (HFS) fed (B/C),
and HFS fed rats treated with each of Quercetin (Q; D), o-Coumaric Acid (CA; E) or berberine (BB; F). HFS induced significant increase in pancreatic islet mass
was non-significantly reduced by each of Q and CA but almost normalized with BB (x400).
Electron micrographs of pancreas from normal diet rats revealed healthy β-cells and organelles (nucleus, rough endoplasmic reticulum, mitochondria and Golgi complex) with a central dense core of typical insulin secretory granules (Figure 2A). HFS-fed rats showed β-cell containing degenerated granules of electron dense core with electron lucent halo (Figure 2B). Pancreas from each of Q or CA treatment groups showed β-cells with degenerated electron dense core granules having increased electron lucent halo (Figure 2C and D). Pancreas from BB-treated group showed β-cell with exhaustion and marked degenerative changes in secretory granules (Figure 2E).
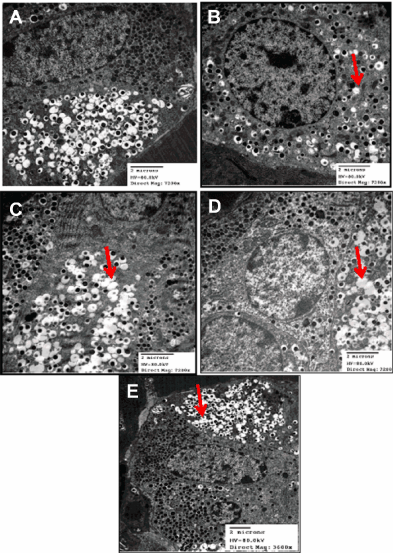
Figure 2: Representative electron micrographs for pancreatic β-cells of healthy normal control (A), High Fat-High Sucrose (HFS) fed (B), and HFS fed rats treated
with each of Quercetin (Q; C), o-Coumaric Acid (CA; D) or Berberine (BB; E). HFS induced degeneration of β-cells electron dense core granules having electron
lucent halo that was slightly ameliorated with the phytochemicals.
Discussion
The increased incidence of obesity, glucose intolerance and IR are major risk factors for type 2 diabetes with a consequent high mortality rate from its cardiovascular complications [37,38]. Current worldwide investigations aim at developing alternative therapeutic phytochemicals that would be more efficacious in counteracting insulin resistance with lesser side effects. The present study is a participation in such efforts studying the potential therapeutic benefits of each of Q, CA and BB against experimental feeding of HFS diet-induced β-cells adverse changes and its consequent alterations in glycemic control indices. Treatment with BB induced superior normalization as compared to each of Q and CA. The significant increase of serum glucose and insulin levels of HFS-fed rats in the present study was previously reported in mice model [39].
HFS diet is associated with pancreatic fatty infiltration resulting in increased insulin levels in obese non-diabetic humans due to impaired β-cell function [40,41]. Impairment in regulation of glucose transporter and pancreatic β-cell cAMP as well as mitochondrial dysfunction, together with decreased adiponectin levels [42-45] are possible pathogenetic mechanisms for the HFS diet-induced insulin insensitivity in the current study. Oral treatment with Q, CA or BB induced significant decreases in serum glucose and insulin levels in accordance with previous researchers [27,46,47]. Stimulation of glycogenesis, up-regulation of key signaling proteins in insulin receptor-dependent pathways, reduction in fat stores and adjustment of leptin and adiponectin levels are potential mechanisms that improved insulin sensitivity following dietary supplementation of these phytochemicals [48].
The present β-cell histo-architectural change in HFS-fed rats was similar to previous findings [49]. Saturated fatty acids inhibit insulin signaling in liver, muscle, and fat cells [50-52] and induce β-cell lipotoxicity via pathways involving endoplasmic reticulum stress and generation of reactive oxygen species [53,54]. However, such insulin resistance mechanisms could be safeguarding measures against energy surplus-induced tissue damage [6].
BB supplementation is cytoprotective through increasing antioxidant enzyme activity and decreasing lipid peroxidation along with enhancing the regenerative capacity of β-cell [55]. Seemingly, its administration led to higher insulin-secreting ability of β-cell as manifested by high increment in the number of electron dense core and limited expansion in the size of pancreatic islet than Q and CA in our study. Flavonoids increase β-cell mass by inhibiting apoptosis and/or promoting proliferation of β-cells [56]. The lower cytoprotective efficacy of CA in HFS models as compared to its p-isomer, p-coumaric acid raises the importance of stereoisomerism of these two compounds. p-Coumaric acid has potent free radical scavenging activity and redox potential [57-59]. Similarly, we had shown that Q - as compared to BB and CA, failed to attenuate the deleterious effect of HFS-induced non-alcoholic fatty liver working through upregulating the peroxisome proliferator-activated receptor-γ, a master adipocyte metabolic regulator [29].
Conclusion
Each of the studied complementary phytochemical normalized the glycemic control indices in HFS diet-induced IR. In this model, BB was the most efficient anti-metabolic syndrome and pancreatic β-cell cytoprotectant. Further studies are currently planned to examine the potential cytokine, transcriptional and epigenetic molecular mechanistic pathway involved in the cytoprotective effects of these compounds particularly for BB that showed better potency and possible distinct mechanisms of action [29,60,61].
References
- Gupta OT. Syndrome X (Metabolic Syndrome) for Generation Z! Why? Metabolic Syndrome and Related disorders. 2015; 13: 193-194.
- Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clinical Investigation. 2013; 123: 2764-2772.
- Boden G, Shulman G. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. European J. Clinical Investigation. 2002; 32: 14-23.
- Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends in Endocrinology & Metabolism. 2003; 14: 137-145.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocrine-Related Cancer. 2009; 16: 1103-1123.
- Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015; 64: 673-686.
- Srinivasan K, Ramarao P. Animal model in type 2 diabetes research: An overview. Indian J Medical Research. 2007; 125: 451-472.
- Shanik MH, Xu Y,škrha J, Dankner R, Zick Y, et al. Insulin resistance and hyperinsulinemia is hyperinsulinemia the cart or the horse? Diabetes Care. 2008; 31: 262-268.
- Ahrén J, Ahrén B, Wierup N. Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets. 2010; 2: 353-356.
- Kahn S. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003; 46: 3-19.
- Mukherjee B, Hossain CM, Mondal L, Paul P, Ghosh MK. Obesity and insulin resistance: An abridged molecular correlation. Lipid Insights. 2013; 6: 1-11.
- Panchal SK, Poudyal H, Iyer A, Nazer R, Alam A, Diwan V, et al. High-carbohydrate high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovascular Pharmacology. 2011; 57: 51-64.
- Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013; 58: 1632-1643.
- Flanagan AM, Brown JL, Santiago CA, Aad PY, Spicer LJ, Spicer MT. High-fat diets promote insulin resistance through cytokine gene expression in growing female rats. J Nutritional Biochemistry. 2008; 19: 505-513.
- Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012; 7: e39837.
- Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clinical Biochemistry and Nutrition. 2010; 46: 212-223.
- DiNardo MM, Gibson JM, Siminerio L, Morell AR, Lee ES. Complementary and alternative medicine in diabetes care. Curr Diab Rep. 2012; 12: 749-761.
- Perez-Vizcaino F, Duarte J, Andriantsitohaina R. Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radical Research. 2006; 40: 1054-1065.
- de Almeida Melo E, Mancini Filho J, Barbosa Guerra N. Characterization of antioxidant compounds in aqueous coriander extract (Coriandrum sativum L.). LWT-Food Science and Technology. 2005; 38: 15-19.
- Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008; 16: 2081-2087.
- Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Separation Science. 2007; 30: 3268-3295.
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Product Reports. 2009; 26: 1001-1043.
- Cheng Z, Pang T, Gu M, Gao A-H, Xie CM, Li JY, et al. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochimica et Biophysica Acta (BBA)-General Subjects. 2006; 1760: 1682-1689.
- Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiology-Endocrinology and Metabolism. 2008; 294: 148-156.
- Hsu CL, Huang SL, Yen GC. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 preadipocytes in relation to their antioxidant activity. J Agric Food Chem. 2006; 54: 4191-4197.
- Hsu CL, Yen GC. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem. 2007; 55: 8404-8410.
- Hsu CL, Wu CH, Huang SL, Yen GC. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J Agric Food Chem. 2009; 57: 425-431.
- Andersen C, Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and adipogenesis. Biofactors. 2010; 36: 415-422.
- Ragab SM, Elghaffar SKA, El-Metwally TH, Badr G, Mahmoud MH, Omar HM. Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: The ameliorative role of three natural compounds. Lipids in Health and Disease. 2015; 14: 1-11.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412-419.
- Lin R-T, Tzeng C-Y, Lee Y-C, Ho W-J, Cheng J-T, Jaung-Geng Lin, et al. Acute effect of electroacupuncture at the Zusanli acupoints on decreasing insulin resistance as shown by lowering plasma free fatty acid levels in steroid-background male rats. BMC Complementary and Alternative Medicine. 2009; 9: 26; 1-9.
- Harishankar N, Vajreswari A, Giridharan NV. WNIN/GR-Ob - An insulin-resistant obese rat model from inbred WNIN strain. Indian J Med Res. 2011; 134: 320-329.
- Kang L, Chen CH, Cheng YC, Chang CH, Lee CT, Chang JK, et al. Glucosamine-induced insulin resistance in ovariectomized rats is relevant to decreasing the expression of glucose transport protein subtype 4 in the skeletal muscle and in increasing the size of pancreatic islets. Menopause. 2012; 19: 496-502.
- Patarroa RS, Lautt WW, Macedo MP. Assessment of methods and indexes of insulin sensitivity. Rev Port Endocrinol Diabetes Metab. 2014; 9: 65-73.
- Radziuk J. Homeostastic model assessment and insulin sensitivity/resistance. Diabetes. 2014; 63: 1850-1854.
- Eltony SA, Elmottaleb NA, Gomaa AM, Anwar MM, El-Metwally TH. Effect of All-trans Retinoic Acid on the Pancreas of Streptozotocin-Induced Diabetic Rat. Anat Rec (Hoboken). 2016; 299: 334-351.
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome a summary of the evidence. Diabetes Care. 2005; 28: 1769-1778.
- Kilpi F, Webber L, Musaigner A, Aitsi-Selmi A, Marsh T, et al. Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutrition. 2014; 17: 1078-1086.
- Yang Z-H, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 2012; 4: 32; 1-10.
- Oakes ND, Bell KS, Furler SM, Camilleri S, Saha AK, Ruderman NB, et al. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997; 46: 2022-2028.
- Zhao Z-z, Xin L-l, Xia J-h, Yang S-l, Chen Y-x, Li K. Long-term high-fat high-sucrose diet promotes enlarged islets and β-cell damage by oxidative stress in Bama Minipigs. Pancreas. 2015; 44: 888-895.
- Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000; 49: 1700-1708.
- Walz HA, Härndahl L, Wierup N, Zmuda-Trzebiatowska E, Svennelid F, Manganiello VC, et al. Early and rapid development of insulin resistance, islet dysfunction and glucose intolerance after high-fat feeding in mice overexpressing phosphodiesterase 3B. J Endocrinology. 2006; 189: 629-641.
- Akagiri S, Naito Y, Ichikawa H, Mizushima K, Takagi T, Handa O, et al. A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clinical Biochemistry and Nutrition. 2008; 42: 150-157.
- de Wilde J, Mohren R, van den Berg S, Boekschoten M, Willems-Van Dijk K, de Groot P, et al. Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiological Genomics. 2008; 32: 360-369.
- Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western style diet in C57/BL6J mice. Molecular Nutrition & Food Research. 2011; 55: 530-540.
- Yang J, Yin J, Gao H, Xu L, Wang Y, Lu Xu et al. Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evidence-Based Complementary and Alternative Medicine. 2012; 1-9.
- Xie X, Li W, Lan T, Liu W, Peng J, Huang K, et al. Berberine ameliorates hyperglycemia in alloxan-induced diabetic C57BL/6 mice through activation of Akt signaling pathway. Endocrine J. 2011; 58: 761-768.
- Abdelmeguid NE, Fakhoury R, Kamal SM, AL Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010; 2: 256-266.
- Dey D, Pal BC, Biswas T, Roy SS, Bandyopadhyay A, Mandal SK, et al. A Lupinoside prevented fatty acid induced inhibition of insulin sensitivity in 3T3 L1 adipocytes. Molecular and Cellular Biochemistry. 2007; 300: 149-157.
- Hommelberg PP, Plat J, Langen RC, Schols AM, Mensink RP. Fatty acid-induced NF-κB activation and insulin resistance in skeletal muscle are chain length dependent. Am J Physiology-Endocrinology and Metabolism. 2009; 296: 114-120.
- Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biological Chemistry. 2009; 284: 14809-14818.
- Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of type 2 diabetes. Biochemical Society Transactions. 2008; 36: 348-352.
- Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends in Endocrinology & Metabolism. 2011; 22: 266-274.
- Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, et al. Protective effect of berberine on beta cells in streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. European J Pharmacology. 2009; 606: 262-268.
- Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr Biochem. 2013; 24: 1777-1789.
- Simić A, Manojlović D,šegan D, Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007; 12: 2327-2340.
- Shairibha SR, Rajadurai M. Anti-diabetic effect of p-coumaric acid on lipid peroxidation, antioxidant status and histopathological examinations in streptozotocin-induced diabetic rats. Int J Int sci Inn Tech Sec B. 2014; 3: 1-11.
- Hosseini A, Shafiee-Nick R, Ghorbani A. Pancreatic beta cell protection/regeneration with phytotherapy. Braz J Pharm Sci. 2015; 51: 1-16.
- Atwell LL, Beaver LM, Shannon J, Williams DE, Dashwood RH, Emily Ho. Epigenetic Regulation by Sulforaphane: Opportunities for Breast and Prostate Cancer Chemoprevention. Curr Pharmacol Rep. 2015; 1: 102-111.
- Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet, and cancer: new mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2012; 51: 213-230.
Citation: Hossam El-Din M Omar, Sohair MM Ragaa, Sary Kh Abd Elghaffar, Abdulrahman A Alduraywish and Tarek H El-Metwally. Berberine, Quercetin and O-Coumaric Acid Phytochemicals Ameliorate the Impact of Experimentally Fed High-Fat/High-Sucrose Diet on Pancreas Β-Cells and Glycemic Control Indices. Austin J Endocrinol Diabetes. 2016; 3(2): 1042. ISSN : 2381-9200