
Special Article - Oral Administration of Leptin
Austin J Endocrinol Diabetes. 2016; 3(4): 1051.
Activation of Brown Adipose Tissue by Oral Administration of Leptin
Bendayan M* and Cammisotto PG
Department of Pathology and Cell Biology, University of Montreal, Canada
*Corresponding author: Bendayan M, Department of Pathology and Cell Biology, University of Montreal, C.P. 6128 Succ. Centre Ville 2900 Edouard Mont Petit, Montreal, Quebec, H3C 3J7, Canada
Received: August 18, 2016; Accepted: October 03, 2016; Published: October 04, 2016
Abstract
Upon demonstrating in previous studies that daily oral administration of leptin is able to regulate food intake and reduce body weight in small rodents while controlling appetite in dogs, we assessed in the present study the action of oral leptin on brown adipose tissue. This thermogenic tissue is stimulated by leptin through activation of UCP1. Leptin increases peripheral lipid oxidation with increased lipolysis and decreased fat synthesis leading to reduction of adiposity. Upon force-feeding leptin to gerbils once a day, the animals reduced their food intake and started to lose weight. Morphological and biochemical examination of the interscapular brown adipose tissue revealed significant activation by oral leptin. By the third day of treatment, the brown adipocytes demonstrated a breakdown of the lipid droplets into smaller ones, an increase in the number of mitochondria and their cristae, while nuclei with extended dispersed chromatin moved towards the center of the cells. By day ten of treatment, the number of lipid droplets decreased being degrade by phagolysosomal structures. Mitochondrial UCP1, key protein in thermogenesis, as well as several enzymes essential to sustain energy metabolism of activated BAT were significantly increased. Thus oral leptin mimics all activities of the endogenous one; it reduces food intake and activates BAT thermogenic activity, thus contributing to the rapid reduction in body weight and adiposity.
Keywords: Oral leptin; Brown adipose tissue; UCP1; Lipolysis
Introduction
In previous series of studies we have demonstrated that in response to food intake, leptin secretion originating from the gastric mucosa is stimulated. At mealtime, gastric chief cells process leptin through regulated secretion [1]. The release of leptin takes place concurrently with other gastric proteins such as pepsinogen and lipase and corresponds to its exocrine secretion into the gastric cavity. As an integral part of the gastric juice, leptin requires protection from early degradation. This is carried out by a chaperon, the soluble isoform of its own receptor [2,3]. A complex leptin-leptin receptor is formed in the chief cell secretory granules prior its discharge into the gastric cavity. The complex is then vehiculated towards the duodenal lumen by the gastric juice. Transmembrane leptin receptors, present on the enterocyte luminal brush border membrane, bind leptin and internalize it towards the Golgi apparatus [3,4]. Upon an intricate transcytotic path, a leptin-leptin receptor complex is released towards the baso-lateral space of the duodenal mucosa and reaches circulation to be carried to the hypothalamic target cells where it acts as a satiety factor [4,5]. On the other hand and concurrently, leptin is continuously synthesized and secreted through a constitutive pathway by white adipocytes [5-7]. This adipocyte-secreted leptin plays important roles not only for the control of appetite but also for regulating energy expenditure [8-13].
Presence of leptin in the gastric cavity led us to propose the oral administration of leptin. Indeed leptin given orally should be very effective in triggering early satiety and could thus control our tendency for overeating. Obesity has become a very serious problem in our western society leading to major health problems [14-16]. Several physiological and psychological issues bring humans to the trend of overeating which in turn gives rise to leptin resistance that exacerbates our system leading to morbid obesity [17,18]. There is a crucial need for safe and efficient approaches to control our food intake. Our studies on leptin secretion by the gastric mucosa led us to envision the prospect of having an oral medication of leptin. Exogenous leptin would undertake the same physiological path as the endogenous one from the gastric cavity into circulation and would reach the target hypothalamic cells to exert its physiological actions [19].
We have previously designed a vehicule capable to administer leptin orally [20]. This vehicle protects the hormone while in transit in the gastric cavity and promotes its absorption by the duodenal mucosa [20]. We reported that once administrated orally to mice, exogenous leptin is transferred very efficiently to blood circulation in a matter of minutes [20]. Oral leptin is able to control food intake and to induce loss of body weight [20]. The efficiency of oral leptin was found to be more striking when given to leptin-deficient ob/ob obese mice [20]. In addition to significantly reducing their food intake, the animals were losing so much body weight that we had to end the experiments [20]. The fact that the ob/ob mice lack leptin but do express leptin receptors at the surface of their cells [21-23], allowed for oral leptin to restore circulating levels of leptin and correct the over-eating problem in these leptin-deficient animals [20]. On the other hand, experiments performed with db/db mice lacking the leptin receptor [21-23], demonstrated that oral leptin administration is not effective [20]. For oral leptin to be efficient, expression of the corresponding leptin-receptor at the plasma membrane of the target cells is essential.
Further work confirmed the efficiency of oral administered leptin. Experiments were carried out on large animals, namely dogs [24]. For such studies, a leptin pill was designed. Human leptin was inserted in a capsule together with components that protect leptin from early degradation during transit in the digestive track and promote its absorption by intestinal cells [24]. As it occurred in rodents, leptin given orally to dogs was able to significantly reduce amounts of food ingested by the animals. Efficiency of the treatment reached 60% in some animals. Treatments were by far more efficient in the mornings that in the afternoons. Correlations between amounts of circulating oral leptin and amounts of food intake were highly significant which indicates that the effectiveness of the treatment is related to the efficiency for leptin to cross the intestinal wall rather than the leptin action by itself [24].
Leptin, a 16kd peptide, regulates adiposity and body weight by controlling food intake and energy homeostasis through its membrane receptor at the level of the target hypothalamic cells and those of some peripheral tissues such as the Brown Adipose Tissue (BAT) [25]. Brown adipocytes play crucial roles in thermogenesis; property conferred by a particular mitochondrial protein, the Uncoupling Protein-1 (UCP1) [26-30]. BAT is found in abundance in particular anatomical sites mainly in small mammals, in hibernating animals, in newborns of large animals and humans [30-33]. In addition to the large number of small lipid droplets the activated brown adipocyte displays numerous mitochondria packed with well developed cristae. Leptin stimulates brown adipocytes through activation of UCP1 [31,34]. The activation of this protein represents a key player in the nonshivering thermogenesis activity for cold acclimation. Leptin increases peripheral lipid oxidation with increased lipolysis and decreased fat synthesis leading to a rapid reduction in body weight and adiposity [35].
In the present study we have evaluated the response of BAT to oral administrated leptin. Gerbils were force-fed daily with leptin and their brown adipose tissue was examined periodically by morphological approach and analysed for the expression of various enzymes and mitochondrial proteins. Results have shown that oral leptin not only reduces food intake in the gerbil but it also activates its brown adipocytes with raise in the number of mitochondria cristae, increases in the expression of various mitochondrial proteins and fragmentation followed by disappearance of the lipids droplets.
Oral leptin mimics all physiological activities of endogenous leptin ; not only it reduces food intake leading to loss of body weight but it also acts on adipose tissue by stimulating lipid oxidation increasing lipolysis and decreasing fat synthesis, overall contributing to a rapid reduction of adiposity.
Material and Methods and Results
Thirteen adult male gerbils weighing an average of 75g were used for this study. After a few days of acclimation 8 animals were force-fed with 100 μg of leptin in the appropriate vehicle, once a day for a period of 11 days. Five control animals were force-fed with the vehicule alone. Based on previous results obtained on mice [20], 100 μg of mouse recombinant leptin (R&D Systems Inc. Minneapolis, MN, USA) were administered orally, dissolved in 0.5 ml of vehicle which composition included 2000 Kallikrein Inhibitor Units (KIU) of aprotinin (Trasylol, Bayer, Leverkusen, FRG) and 5 mg of sodium deoxycholate (22mmol/l) (Sigma St-Louis, Mo, USA) in bicarbonate buffer pH 9. Aprotinin reduces leptin degradation in the gastric cavity while sodium deoxycholate enhances duodenal absorption [36-38]. Food intake and body weight were monitored daily. Results are reported in Figures 1&2. Figure 1 demonstrates that oral leptin reduces the daily food intake by 55% as soon as the leptin treatment was initiated. Body weight show steady decreases when compared to vehicle-fed control animals already by the second day of treatment (Figure 2). Body weight continued to decrease as long as the treatment with oral leptin was maintained (Figure 2). These results are similar to those previously obtained on mice [20].
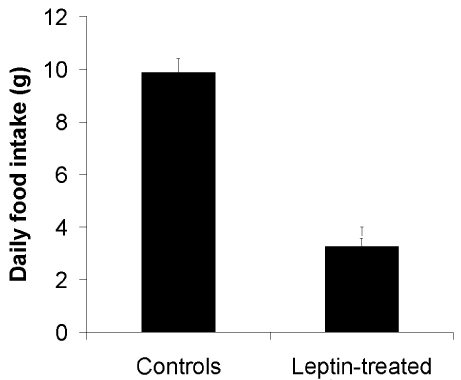
Figure 1: Average food intake per day by control (vehicule-fed) (N=5) and
leptin-treated gerbils. (N=8).

Figure 2: Body weight of non-treated (N=5) and leptin-treated (N=8) gerbils.
Morphological studies on BAT were carried out upon oral administration of leptin. We chose to examine the interscapular brown adipose tissue. Tissues were sampled and fixed by immersion in Bouins’ fixative prior processing them for embedding in paraffin according to routine histological techniques. For electron microscopy, small fragments of BAT were fixed by immersion in 1% glutaraldehyde in 0.1M phosphate buffer for 2 hours, post-fixed with 1% osmium tetroxide at 4ºC and upon dehydration in graded ethanol and propylene oxide embedded in Epon. Tissue thin sections (70nm) were performed with an ultra-microtome and upon staining them with uranyl acetate and lead citrate, they were examined with a Philips electron microscope.
In control vehicule-fed animals, BAT appears light colored. Upon stimulation by leptin it turned to a rather brownish color which we know to be due to proliferation and activation of mitochondria as well as disappearance of the lipid deposits. Also, the dense vascularisation in the connective tissue must contribute to the appearance of BAT. Morphological examination of the BAT by light microscopy (Figure 3) demonstrated the presence of cells containing large lipid droplets resembling in many instances those of the white adipose tissue. Interstitial connective tissue appeared abundant with significant vascularisation (Figure 3). By electron microscopy nonstimulated brown adipocytes displayed one or two large lipid droplets surrounded by a thick rim of cytoplasm filled with mitochondria. The large nucleus was located in the peripheral cytoplasm (Figure 4). The cells were closely related to numerous blood capillaries (Figure 4). This contrasts with the features of the white adipocytes which display a single large lipid droplet surrounded by a very thin peripheral cytoplasmic rim with few mitochondria and a flattened nucleus.
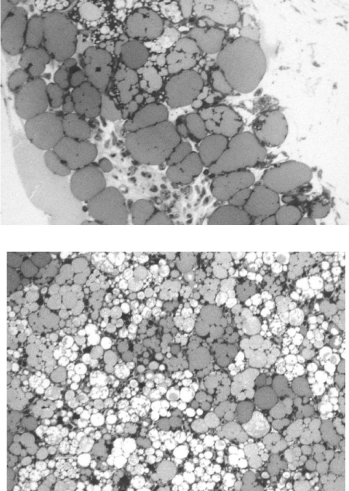
Figure 3: BAT from non-treated (top figure) and 3 days leptin-treated (bottom
figure) animals. Tissue from leptin-treated animals displays a higher number
of lipid droplets much smaller in size.
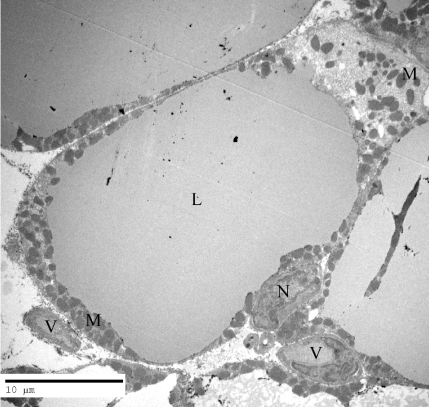
Figure 4: Electron micrograph of a brown adipocyte of a control animal. It
displays a large Lipid droplet (L) surrounded by a wide rim of cytoplasm filled
with Mitochondria (M). The Nucleus (N) is located at the periphery of the
cell. Numerous blood Vessels (V) are located close to the adipocyte in the
connective tissue.
Upon oral administration of leptin, BAT is activated and the morphology of its cells changes drastically as early as after three days of treatment. By light microscopy the cells appeared to be filled with numerous lipid droplets much smaller in size than those found in resting cells (Figure 3). Tissue appears more compact with increased vascularisation. By electron microscopy we detected several changes that evolved along with the length of the oral leptin treatment. Upon 3 days of leptin stimulation the morphology of the brown adipocyte differs drastically from that of the resting cell. The single large lipid droplet had fragmented into numerous smaller ones (Figure 5). These new smaller lipid droplets fill up the cytoplasm. The peripheral nucleus moved towards the center of the cell and its dense chromatin evolved towards the dispersed type. Mitochondria increased in number with a darker aspect. In fact, the number of cristea within each mitochondrion increased drastically and the mitochondria matrix was denser (Figure 6). Number of blood capillaries seemed to have increased and they moved close to the adipocyte plasma membrane (Figure 5).

Figure 5: Low magnification electron micrograph, illustrating the brown
adipose tissue of an animal receiving oral leptin for a period of three days.
Many changes are clearly seen. The large lipid droplets have fragmented into
numerous smaller ones (L). The Nuclei (N) have moved towards the centre
of the cells and the blood Vessels (V) are numerous and in close contact with
the adipocytes.
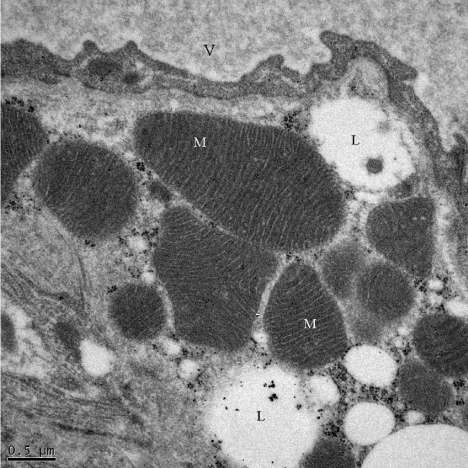
Figure 6: Brown adipocyte Mitochondria (M) from a three days leptin-treated
animal. Mitochondria are numerous and display a very large number of
cristea, Mitochondria matrices appear very dense. V: blood Vessel; L: Lipid
droplets.
Morphology of the brown adipocyte continued to evolve as the treatment with leptin was prolonged. Upon ten days of treatment, the aspect of the brown adipocytes was quite different from that of the resting cells or even from the short-term activated ones (Figure 7). While nuclei were still centrally located and the number of mitochondria remained very high, the number of lipid droplets decreased drastically and those still present were surrounded by phagolysosomal like structures (Figure 7). The presence of these phagolysosomes indicates dynamic digestive activities. Nuclei matrix became quite clear with very small nucleoli; progression towards pyknosis and cell death was noted. Blood vessels still very abundant remained in close contact with the adipocyte cell membrane.

Figure 7: High magnification of a cytoplasmic area of a brown adipocyte
from an eight days leptin-treated animal. The number of Lipid droplets
(L) has diminished drastically and the few remaining are surrounded by
Phagolysosomal structures (PL). Mitochondria (M) remain numerous. N:
Nucleus; V: blood Vessel.
Biochemical assessment of the activated brown adipose tissue was performed through analysis of different proteins and mitochondrial enzymes. BAT from control and leptin-administered animals were sampled. The first protein considered was indeed the UCP-1 (or thermogenin) which is found exclusively in BAT [27,34,39]. It is a transmembrane protein of the inner mitochondria membrane. Protons present in the inter-mitochondrial membranes are channelled through UCP-1 into the mitochondrial matrix. This results in a drastic loss of energy in the form of heat, concomitant with a decrease in ATP synthesis. An increase in the UCP-1 protein is characteristic of activated BAT [27]. UCP-1 in our gerbil BAT was determined by immunoblotting and referred to ß-actin levels. Oral administration of leptin led to significant increases in UCP-1 confirming that oral leptin was able to trigger mitochondria activation (Figure 8). Lipogenesis in brown adipocytes requires activation of mitochondria enzymes with a proper supply of NADPH for the synthesis of fatty acids. This is ensured by the malic enzyme and the glucose-6P dehydrogenase. Malic enzyme was measured by generation of NADPH in the presence of L-Malate [40]. Figure 9 demonstrates that the malic enzyme is highly enhanced upon oral leptin administration. Similar results were obtained for glucose-6P dehydrogenase (Figure 9) which was assessed by incubation of glucose and ATP to generate glucose- 6P followed by a NADP and glucose-6Pdehydrogenase reaction to produce NADPH [41]. Glucose-6P dehydrogenase converts glucose- 6P into 6P-D-gluconate with generation of NADPH [40]. Again the glucose-6P dehydrogenase was found to be increased under oral administration of leptin (Figure 9).
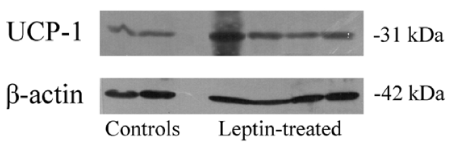
Figure 8: UCP1 (or thermogenin) is drastically enhanced in brown adipose
tissue of leptin treated animals.
Besides lipids, glucose is an important fuel during BAT activation with a high capacity of glycolysis. The rate of glycolysis was assessed by measuring the activities of glucokinase and pyruvate kinase. Pyruvate kinase activity was measured by conversion of phosphoenolpyruvate to pyruvate in the presence of ADP followed by pyruvate to lactate in the presence of NADH and LDH [42]. Glucokinase and pyruvate kinase activities were both increased in BAT upon oral administration of leptin (Figure 9). Finally, cytochrome c oxidase being a key enzyme of the mitochondrial respiratory chain was also assessed using the criteria of its oxidation of vitamin C [43]. And again upon oral administration of leptin, cytochrome c oxidase was found to be significantly increased (Figure 9).
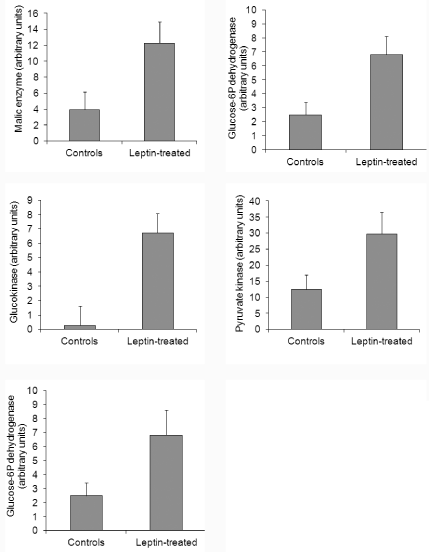
Figure 9: Levels of various enzymes of brown adipose tissue from control
and leptin-treated animals.
All along the study, comparative assessments were carried out with tissues from control animals and results were statistically evaluated with standard errors to the means and Student t-test analysis.
Discussion
We previously established that the gastric mucosa secretes leptin at time of food intake [1]. This secretion takes place concomitantly with other gastric proteins such as pepsinogen and lipase, leptin being an integral part of the gastric juice. Leptin secreted by the gastric chief cells represents an unusual example of the exocrine secretion of a hormone. Due to the drastic conditions existing within the gastric juice and leptin being a small peptide, survival of leptin requires protection. This is provided by a chaperon. We have demonstrated that leptin is secreted associated to the soluble isoform of its receptor [2,5]. The complex leptin-leptin receptor formed within the secretory granules of the gastric chief cells is released into the gastric cavity by exocytosis. The leptin-leptin receptor complex resists the harsh conditions of the gastric juice and is vehiculated towards the duodenum. Once in the intestinal lumen, leptin interacts with its integral transmembrane receptor present on the apical brush border membrane of the intestinal cells leading to its internalization by endocytosis [4,5,19]. From the endosomal compartment it is channeled to the Golgi apparatus where it gets complexed again to its soluble receptor before being released towards the interstitial tissue to reach blood circulation [5]. Thus, leptin secreted by the gastric mucosa into the gastric juice finds its way to reach circulation across the intestinal cells.
The fact that a peptide or a protein present in the intestinal lumen reaches blood circulation in an intact and functional form is not a unique situation. We have previously reported that some pancreatic proteins secreted in an exocrine fashion into the pancreatic juice, cross the intestinal wall to reach blood circulation [44-46]. Indeed, pancreatic lipase as well as bile salt dependant lipase, secreted by the exocrine pancreas into the pancreatic juice, crosses the intestinal cells through an elaborate transcytotic pathway to reach blood circulation [44-46] where they play key roles.
It is also important to underline that formation of the leptinleptin receptor complex is crucial for the physiological activity of this hormone since both leptin peptides, the one originating from the gastric mucosa as well as that of the adipose tissue, circulate in the blood in their complexed form, associated to the soluble isoform of their receptor [5]. We herein emphasize the fact that the circulating physiological hormone is not the peptide by itself, but rather the complex leptin-leptin receptor.
The presence of leptin in the gastric cavity as an integral part of the gastric juice motivated us to propose that leptin could be administered orally. Oral leptin would reach the gastric cavity and provided degradation by the gastric juice is prevented, it would reach the intestinal lumen. It would then be internalized by enterocytes and complexed to its soluble receptor prior being discharged on the basal interstitial space to reach target cells via blood circulation [5,19]. Experiments were performed to establish this path. Leptin forcedfed to normal mice, was found to efficiently reach blood circulation through the intestinal wall in matters of minutes and able to decrease food intake to such an extent that the animals start losing weight [20]. The efficiency of oral leptin was even more drastic when administered to ob/ob mice [20]. These mice, genetically deficient of leptin, become rapidly obese [21,22]. Daily oral administration of leptin was able to control their food intake and even induce major loss of their body weight [20].
Further studies were performed with small mammals [20] including the rat and the gerbil (present study) as well as with large animals such as the dog [24]. In all instances the efficiency of oral leptin to decrease food intake was established provided that major degradation of the leptin peptide in the gastric cavity is prevented. Two approaches to reach this objective; the most direct and efficient one would be to bind leptin to the soluble isoform of its receptor as it occurs in vivo. Alternatively, we choose to prevent major degradation by using a protease inhibitor [20]. This was found to be very efficient since significant amounts of leptin reach blood circulation within the first 15 minutes [20]. Working with obese mice such as the ob/ob and the db/db mice allowed us to establish that oral administered leptin is very efficient in reaching the hypothalamic targets cells and inducing significant reduction of food intake provided that the transmembrane leptin receptor is expressed on the plasma membrane of the target cells [20]. In the case of the db/db mice deficient in leptin receptor, oral administered leptin was unable to induce any changes in the feeding behavior [20]. The integrity of the leptin receptor and its expression at the cell plasma membrane are primordial for our treatment with oral leptin to be efficient.
Besides acting on feeding behavior, leptin is known to activate brown adipose tissue [27,39]. In the present study we looked into the activation of BAT by oral leptin. The study was performed on gerbils that present significant amounts of BAT particularly in the interscapular region. Leptin was orally administered daily and the BAT was examined at three different time points: prior leptin administration, 3 and 10 days after daily administration of an amount of leptin that was able to reduce food intake and even decrease body weight. Indeed, immediately upon starting the oral administration of leptin, the animals reduced significantly their food intake and started to lose weight. The loss of body weight resulted from the combination of two actions, the loss of appetite with reduced ingestion of food but also by activation of the BAT. This was demonstrated by morphological and biochemical approaches. Brown adipocytes under resting condition present one or two large lipid droplets surrounded by a large rim of cytoplasm that displays numerous mitochondria and a large, spherical and peripheral located nucleus. In addition, the connective tissue is rich in blood vessels.
Oral administered leptin reaching rapidly blood circulation acted upon these BAT target cells. As soon as three days of daily oral administration of leptin, we were able to demonstrate activation of the BAT. The large lipid droplets broke down into numerous small ones; mitochondria proliferated and the large nuclei became centrally located with developed dispersed chromatin. The mitochondria show increased number of cristae and a dense matrix. We were also able to observe that the numerous capillary blood vessels got closely associated to the adipocytes. Thus activation of lipolysis was triggered by the oral leptin administration. After ten days of treatment, few lipid droplets remained in the adipocytes. They were surrounded by autophagolysosomes that encapsulated them to proceed with lipolysis [47]. Mitochondria remained numerous each displaying a large number of cristae and dense matrices. UCP1 the main protein involved in thermogenesis, present exclusively in BAT mitochondria, was found to be increased demonstrating activation with decrease in ATP synthesis and production of heat. Similarly other mitochondria enzymes involved in lipogenesis were increased confirming again activation of BAT.
Taken together all the data clearly demonstrate that once orally administrated, leptin reaches target tissues through circulation triggering activation of BAT. Besides acting as a satiety hormone reducing appetite and decreasing food intake, leptin stimulates brown adipocytes triggering lipogenesis, stimulating mitochondria and generating large amounts of heat thus contributing to loss of body weight.
Oral administration of leptin should be considered as a very promising opportunity for managing body weight loss in obese patients. Several points should be taken into consideration when bearing in mind oral administration of leptin: 1- protection of the oral administered leptin from early degradation by the gastric juice, 2- the fact that the physiological active circulating factor is not the free leptin peptide but rather the leptin-leptin receptor complex and 3- the leptin hormone does not act only on satiety but also activates lipolysis with heat production. These combined actions lead to major loss of body weight.
Acknowledgment
The authors would like to express their gratitude to Daniel Hofmann and Doctor Ilan Hofmann for their support and enthusiasm. This work was supported by a grant from I-Med Pharma Inc.
References
- Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005; 53: 851-860.
- Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006; 290: 242-249.
- Cammisotto PG, Bendayan M, Sané A, Dominguez M, Garofalo C, Levy E. Receptor-Mediated Transcytosis of Leptin Through Human Intestinal Cells. Int J Cell Biol. 2010; 928169.
- Cammisotto PG, Gingras D, Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol. 2007; 293: 773-779.
- Cammisotto PG, Levy E, Bukowiecki LJ, Bendayan M. Cross-talk between adipose and gastric leptins for the control of food intake and energy metabolism. Prog Histochem Cytochem. 2010; 45: 143-200.
- Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002; 283: 244-250.
- Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M. Leptin biosynthetic pathway in white adipocytes. Biochem Cell Biol. 2006; 84: 207-214.
- Schwartz MN, Porte D Jr. Diabetes, obesity and the brain. Science. 2005; 307: 375-379.
- Schwartz MW, Woods Sc, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000; 404: 661-671.
- Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increases in catecholaminine secretion. Diabetes. 1999; 48:1787-1793.
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995; 269: 546-549.
- Himms-Hagen J. Physiological roles of the leptin endocrine system: differences between mice and humans. Crit Rev Clin Lab Sci. 1999; 36: 575-655.
- Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Semin Vasc Med. 2005; 5: 15-24.
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010; 67: 220-229.
- Hu FB. Epidemiology studies of consequences of obesity. In Obesity epidemiology. Oxford University Press New York. 2008.
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. (Lond). 2011; 35: 891-898.
- Myers MG Jr, Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, et al. Towards a clinical definition of leptin resistance. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012; 15: 150-156.
- Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010; 21: 642-651.
- Bendayan M, Cammisotto P. Oral Leptin Administration: I- Leptin secretion by adipose tissue and gastric mucosa for the control of food intake: A review. Endocrinol Diabetes Submitted. 2016.
- Bendayan M, Cammisotto P. Oral Leptin Administration: II- Control of food intake and body weight in small rodents. Endocrinol Diabetes Submitted. 2016.
- Coleman DL, Hummel KP. Studies with the mutation diabetes in the mouse. Diabetologia. 1967; 3: 238-248.
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978; 14: 141-148.
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950; 41: 317-318.
- Bendayan M, Cammisotto P. Oral Leptin Administration: III- Control of food intake in dogs. Endocrinol Diabetes Submitted. 2016.
- Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997; 100: 2858-2864.
- Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes. (Lond). 2008; 32: 32-38.
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012; 151: 400-413.
- Yi CX, Meyer CW, Jastroch M. Leptin action in brain. How and when it makes fat burn. Mol Metab. 2013; 2: 63-64.
- Commins PM, Watson IC, Frampton, TWG. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependant mechanism in brown adipose tissue. Am J Physiol Endocr Metab. 2001; 280: 372-377.
- Kortelainen ML, Pelletier G, Ricquier D, Bukowiecki LJ. Immunohistochemical detection of human brown adipose tissue uncoupling protein in an autopsy series. J Histochem Cytochem. 1993, 41: 759-764.
- Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972; 112: 35-39.
- Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004; 84: 277-359.
- Géloën A, Collet AJ, Guay G, Bukowiecki LJ. In vivo differentiation of brown adipocytes in adult mice: an electron microscopic study. Am J Anat. 1990; 188: 366-372.
- Cadrin M, Tolszczuk M, Guy J, Pelletier G, Freeman KB, Bukowiecki LJ. Immunohistochemical identification of the uncoupling protein in rat brown adipose tissue. J Histochem Cytochem. 1985; 33: 150-154.
- Sarmiento U, Benson B, Kaufman S, Ross L, Qi M, Scully S, et al. Morphologic and molecular changes induced by recombinant human leptin in the white and brown adipose tissues of C57BL/6 mice. Lab Invest. 1997; 77: 243-256.
- Ziv E, Bendayan M. Intestinal absorption of peptides through the enterocytes. Microsc Res Techn. 2000; 49: 346-352.
- Bendayan M, Ziv E, Ben-Sasson R, Bar-On H, Kidron M. Morpho-cytochemical and biochemical evidence for insulin absorption by the rat ileal epithelium. Diabetologia. 1990; 33: 197-204.
- Bendayan M, Ziv E, Gingras D, Ben-Sasson R, Bar-On H, Kidron M. Biochemical and morpho-cytochemical evidence for the intestinal absorption of insulin in control and diabetic rats. Comparison between the effectiveness of duodenal and colon mucosa. Diabetologia. 1994; 37: 119-126.
- Porter RK. A new look at UCP1. Biochem Biophys Acta. 2006; 1757: 446-448.
- Carvalho DS, Negrao N, Bianco AC. Hormonal regulation of malic enzyme and glucose-6-phosphate dehydrogenase in brown adipose tissue. Am J Physiol. 1993; 264: 874-881.
- Postic CL, Leturque A, Printz RL, Maulard P, Loizewu M, Granner DK, et al. Development and regulation of glucose transporter and hexokinase expression in rat. Am J Physiol. 1994; 266: 548-559.
- Pon NG, Bondar RJ. A direct spectrophotometric assay for pyruvate kinase. Anal Biochem. 1967; 19: 272-279.
- Wharton DC, Alexander Tzagoloff. Cytochrome oxidase from beef heart mitochondria. Methods in Enzymology. 1967; 10: 245-250.
- Bruneau N, Bendayan M, Gingras D, Ghitescu L, Levy E, Lombardo D. Circulating bile salt-dependent lipase originates from the pancreas via intestinal transcytosis. Gastroenterology. 2003; 124: 470-480.
- Bruneau N, Lombardo D, Levy E, Bendayan M. Roles of molecular chaperones in pancreatic secretion and their involvement in intestinal absorption. Microsc Res Tech. 2000; 49: 329-345.
- Cloutier M, Gingras D, Bendayan M. Internalization and transcytosis of pancreatic enzymes by the intestinal mucosa. J Histochem Cytochem. 2006; 54: 781-794.
- Thiam AR, Farese RV Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nature Rev. 2013; 14: 775-786.