
Research Article
J Endocr Disord. 2014;1(3): 1013.
A Systematic Review and Meta-Analysis of Acarbose in the Treatment of Polycystic Ovary Syndrome
Lihua Wang, Tingting Han, Jiang Yue, Wei Liu and Yaomin Hu*
Department of Endocrinology and Metabolism diseases, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, China
*Corresponding author: Yaomin Hu, Department of Endocrine and Metabolic diseases, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Received: August 18, 2014; Accepted: October 08, 2014; Published: October 10, 2014
Abstract
Objective: To evaluate the efficacy and tolerability of acarbose in the treatment of Polycystic Ovary Syndrome (PCOS).
Methods: We researched the database of PubMed, Biomedical Core Database (BCD), Cochrane library databases, and Chinese Biological Medicine for Randomly Control Trials (RCTs) and analyzed the retrospect data of acarbose in the treatment of PCOS.
Results: There are seven RCTs that met the inclusion criteria. As different control groups (placebo or metformin) have been used in these trials, we separated the data into two independent groups. The dose of acarbose in the treatment groups ranged from 150 to 300 mg/day. VLDL, triglycerides, Luteinizing Hormone (LH), testosterone, and Dehydroepiandrosterone Sulfate (DHEAS) levels decreased, whereas HDL significantly increased in acarbose groups when compared with the placebo groups. Acarbose treatment also improved menstrual frequency and insulin level. We couldn’t find any differences between acarbose group and metformin group in fasting insulin level, BMI, LH, FSH, and testosterone level. However, based on these three RCTs data, the pregnancy rate was higher in acarbose group than that in metformin group (OR=3.02, 95% CI: 1.10-7.71, P=0.02). The gastrointestinal adverse effects of acarbose were found to be higher in treatment groups than those in placebo groups, and similar to or slightly less than those in metformin groups.
Conclusions: Treatment with acarbose, at the dose of 150-300 mg/d, has been shown to improve various clinical manifestations of PCOS and be a safe and effective drug for the treatment of these patients, especially for patients who are intolerant of metformin. However, it may be too early to draw conclusions due to limitations in the currently available data.
Introduction
Polycystic Ovary Syndrome (PCOS), which affects 2.2-10% of reproductive women, is a complex, multisystemic disorder presenting with oligomenorrhea of amenorrhea, infertility, hirsutism, acne, hypersecretion of androgens, and polycystic ovary [1-4]. PCOS is associated with obesity, insulin resistance, hyperinsulinemia, impaired glucose tolerance and disorders of lipid metabolism, suggesting that this subgroup of patients is at risk for the development of type 2diabetes and cardiovascular disease [5]. In addition, endometrium, ovary and breast cancers are also long-term consequences of PCOS [6,7]. Therefore, active invention for PCOS to prevent further problems is deemed to vital.
Insulin resistances, presented as impaired insulin-mediated glucose uptake, are an integral feature of PCOS and have been reported in both obese and non-obese patients [8,9]. It has been shown that hyperinsulinemia may enhance LH release and androgen synthesis and secretion, also inhibit hepatic synthesis of Sex Hormone Binding Globulin (SHBG). Up to 30% of obese PCOS patients showed impaired glucose tolerance and 7.5-10% patients were diagnosed of Type II Diabetes Mellitus (T2DM) screened by Oral Glucose Test (OGTT). Therefore, numbers of medications that improve insulin sensitivity have been used to treat PCOS patients.
Acarbose, and α-glucosidase inhibitor, is a common first-line treatment in T2DM. Acarbose is shown to reduce and slow down the intestinal absoption of glucose, which subsequently minimise the post-prandial rise of blood glucose and insulin concentration. Acarbose does not cause hypoglycemia and its minor gastrointestinal side effects can be prevented by gradual dosage increments. Therefore, acarbose has been used in the management of PCOS in recent years [10].
Recently, several clinical trials have been conducted to investigate the effects of acarbose on PCOS by comparing the effects with placebo or metformin. The objective of this meta-analysis is to pool data from these trials and trying to evaluate the efficacy and tolerability of acarbose in the treatment of PCOS.
Research Design and Methods
A comprehensive and systematic search of published literature for trials of acarbose in the treatment of PCOS was performed during May 2014 using PubMed, Biomedical Core Database (BCD), Cochrane Library Databases, and Chinese Biological Medicine. The search strategy was not limited by year or language of publication. The key words used in this search were acarbose or α-glucosidase inhibitor, and PCOS or polycystic ovary syndrome. Study treatment durations ranged from 3 to 6 months, and the effective dosing ranged from 150 to 300 mg/d. Each of the studies shared fundamental inclusion criteria, including: menstrual disorders (<6 menstruations/12 months), clinical [Ferriman-Gallwey (FG) index ≥8] or laboratory (testosterone >80 ng/dl and/or androstenedione >190 ng/dl) hyperandrogenism and insulin resistance. Alteration of hepatic, renal and thyroid function, presence of congenital adrenal hyperprolactinemia, presence of congenital adrenal hyperplasia, presence of diabetes, and the use of hormonal medications or medications that might interfere with carbohydrate metabolism over the last 6 months were exclusion criterions in this study. Six of seven trials studied patients with obesity.
The efficacy measures in each study were clinical (number of menstrual cycles, FG score, ovulation or pregnancy rate), anthropometric (weight, height, BMI), hormone and metabolic evaluation before and after treatment. Safety measures included incidence of adverse events, such as flatulence and/or diarrhea.
Statistical Analysis
A three-item, 1-5 quality scale to score each report has been used to meet the inclusion criteria. The use of concealment and intention-to-treat analysis was also assessed. Two of the three reviewers made quality assessment. Their disputes were settled by consensus. The analyzed data consisted of group means that were reported in one paper. The results were combined and expressed as Odds Ratio (OR) or Weight Mean Difference (WMD) with 95% confidence intervals (95% CIs) using a Fixed Effect (FE) or Randomized Effect (RE) model, for the studies with sufficient data. Additionally, homogeneity was assessed with I2 statistic and χ2 test. All statistics for meta-analysis were calculated by Revman Manager 5.2 Software (Copenhagen, Denmark). Sensitivity analysis was applied to explore the influence on outcomes via changing effect model or excluding studies with abnormal results.
Results
Description of studies
Seventy citations have been screened for eligible for inclusion in meta-analysis. Sixty-three studies were excluded including duplicated data from different databases or not related (n=55), general reviews of drugs or disease areas (n=5), and self-control studies (n=2). Therefore, seven studies, which met the inclusion criteria, were used in our meta-analysis [11-17]. Six studies and an abstract of one study were published in English. As different drugs were used in control groups, we devided these studies into two groups. The characteristics and methodological quality of the included studies are shown in Table 1. The age of the subjects was not reported in some of the studies.
Trial
Design of trials
Number (T/C)
Age (years, T/C)
Acarbose
dose (mg/d)
Drug of control group (mg/d)
Duration (months)
Quality
Acarbose vs placebo
Ciotta et al. [16]
RCT
15/15
20.5/20.9
300
placebo
3
B
Penna et al. [14]
RCT
13/14
26.7/25.9
150
placebo
6
B
Penna et al. [13]
RCT
13/14
26.7/25.9
150
placebo
6
B
Tugrul et al. [12]
RCT
44/30
27.1
300
placebo
3
B
Acarbose vs Metformin
Sonmez et al. [15]
RCT
15/15
-/-
300
1700
3
B
Hanjalic-beck et al. [11]
RCT
29/27
28.0/28.0
300
2550
3
B
Moini et al. [17]
RCT
24/22
-/-
300
1500
3
B
Table 1: Characteristics and methodological quality of included studies.
Effects of acarbose treatment
Four trials with a total 131 patients were analyzed. There were 72 patients in acarbose groups and 59 patients in control groups. Two trials administrated by Penna [13,14] are same trials but for different purpose.
Body Mass Index (BMI) and Free Glucose (FG) score for both acarbose and control groups are shown in Figure 1. There were no significant differences between these two parameters.
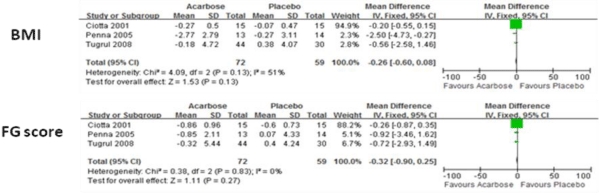
Figure 1: Comparision of efficacy of acarbose group with control group for BMI and FG score.
Figure 2 shows the results of FE models comparing the means of total cholesterol, very low-density lipoprotein cholesterol (VLDL-C), LDL-C, HDL-C, and triglycerides for both treatment and control groups. When compared with placebo, acarbose decreased VLDL-C (P<0.01) and triglyceride levels (P=0.05) but significantly increased HDL-C levels (P<0.01). No significant improvement for total cholesterol has been found.
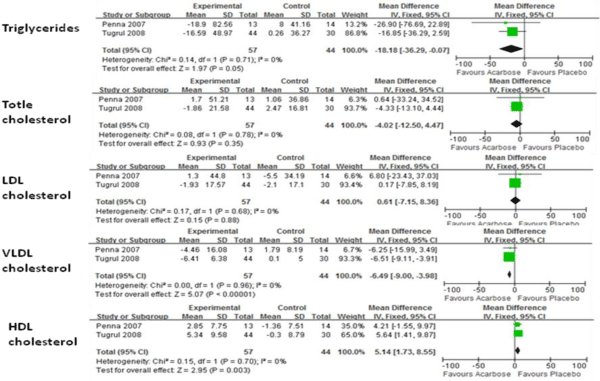
Figure 2: Comparision of efficacy of acarbose group with control group for lipid profile.
Figure 3 shows the results of RE or FE models comparing the means of sex hormone in both groups. When compared with placebo, acarbose significant decreased LH, testosterone, and DHEAS levels (P<0.001). No significant improvements for FSH and SHBG have been found.
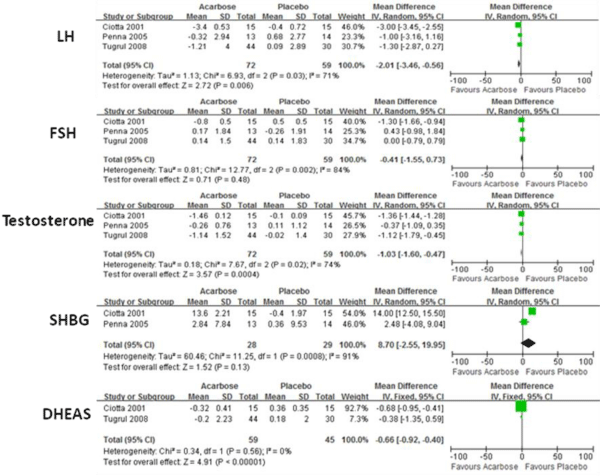
Figure 3: Comparison of efficacy of acarbose group with control group for sex hormone.
Effects of acarbose and metformin treatment
Three trials with a total 138 patients were analyzed. There were 72 patients in acarbose groups and 66 patients in metformin groups.
Figure 4 presents the means of BMI and Fasting Insulin (FINS) for acarbose groups and metformin groups. There were no significant differences for these indexes.
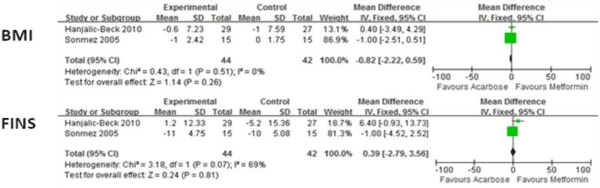
Figure 4: Comparison of efficacy of acarbose group with metformin group for BMI and FINS.
FINS: fasting insulin.
Figure 5 shows the means of LH, FSH and testosterone levels in acarbose groups and metformin groups. There were no significant differences for these indexes, either.
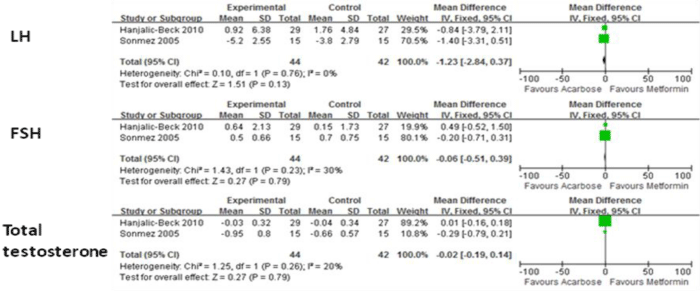
Figure 5: Comparison of efficacy of acarbose group with metformin group for sex hormone.
Figure 6 shows the means of pregnancy rate for acarbose groups and metformin groups. When compared with metformin, acarbose significantly increased pregnancy rate (P=0.02).
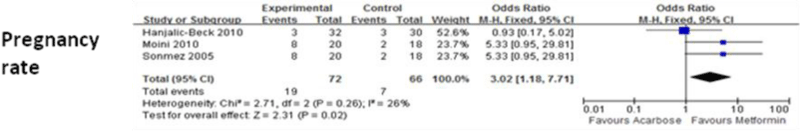
Figure 6: Comparison of efficacy of acarbose group with metformin group for pregnancy rate.
Adverse events
The most widely reported adverse effect of acarbose was related to gastrointestinal side effects, such as abdominal pain, distention, and diarrhea. Six out of thirty-eight women dropped out due to various side effects in one study. In remaining studies, side effects were minor or could be tolerated by slowly increasing the dose of acarbose. In summary, the gastrointestinal adverse effects of acarbose were found to be higher than that of placebo and similar to or lighter than those of metformin.
Sensitivity analysis
As the FE and RE models produced similar estimates of treatment effects, the outcomes appear to be stable.
Discussion
PCOS adversely affects women throughout their lifetime, leading to diabetes, metabolic syndrome and cardiovascular disease. Although PCOS is heterogeneous, insulin resistance and hyperinsulinemia are common and occur independently of obesity [18]. Insulin-resistant hyperinsulinemic and hyperandrogenic states are two main factors fundamentally related to PCOS. Their relationship is considered to be very close. Hyperinsulinemia, acts as a congonadotropin with Luteinizing Hormone (LH), increases androgen production by theca cells and reduces hepaic production of sex hormone binding globulin, resulting in higher concentrtion of free androgens and causing hyperandrogenic state [19]. Hyperinsulinemia also increases levels of circulating Insulin Like Growth Factor 1 (IGF- 1) by inhibiting the production of IGFBP-1. IGF-1 then binds to its receptor in the ovary and stimulates androgen production [20]. In obese patients with PCOS, the activity of cytochrome P450 17α, which mostly expressed in ovaries and adrenal glands, are higher and the conversion of estrone by aromatase in peripheral adipose tissue are greater [21]. Furthermore, a reduction in serum insulin level leads to a decreased androgen level. Insulin sensitizers decrease hyperinsulinemia by increasing insulin sensitivity. Therefore, they have been proposed as a therapy for the treatment of PCOS with fasting or stimulated hyperinsulinemia. Metformin is the oldest and still the most used insulin sensitizer worldwide. In women with PCOS, metformin decreases insulin, testosterone and LH levels when administered at doses of up to 1500 mg/day [21]. Meta-analysis showed that metformin is effective in achieving ovulation in women of PCOS when compared to placebo or clomiphene alone. However, gastrointestinal side effects of metformin often limit its use, and other agents that improve insulin sensitivity have also been used for PCOS patients. Acarbose, an alpha-glucosidase inhibitor, works at the brush border of the small intestine to reduce glucose absorption in the gut and subsequently lower postprandial glucose and insulin levels. Ciotta et al. [16] first reported the use of acarbose in patients with PCOS in 2001. They found that acarbose could reduce the androgenic activity and make the menstrual cycle regular. To date, only a few RCTs could be retrieved to address the effects of acarbose on women with PCOS. In this meta-analysis, 7 RCTs and 269 patients were included. Due to different control groups, we have to analyze in two different parts to reduce bias of the results.
When compared with placebo, acarbose exhibited obvious benefits on lowering LH, testosterone and DHEAS levels of PCOS patients. In contrast, changes in SHBG and FG score were not significant. Two of these studies also observed an increased chance of menstrual regularity. As we all know, increased serum LH concentrations or LH/FSH ratios, associated with the continuation of the anovulatory state, have long been recognized as one of the main causes of PCOS. However, it is not included into the current Rotterdam diagnostic criteria for PCOS [22]. These data indicated that acarbose could improve not only hyperandrogenism, but also the disorders of gonadal axis. We couldn’t analyze the role of acarbose on insulin resistance and hyperinsulinemia, because of different evaluation methods were used in these studies. In two of the three studies, a significant reduction in the basal insulin level or insulin response to glucose load was observed. Penna et al. failed to detect a significant change in insulinaemic response after glucide stimulation. However, they found that acarbose was able to reduce body weights [14]. In addition, acarbose can significantly improve lipid profile, decrease VLDL levels and increase HDL levels Furthermore, its beneficial effect on blood pressure and P-selectin were also observed by Penna et al, indicating that acarbose might be an important tool in the prevention of cardiovascular disease in PCOS patients. Many studies have indicated that metformin reduces appetite, improves glucose uptake by peripheral tissues, decreases hepatic gluconeogenesis and fatty acid synthesis, alleviates systemic inflammation, and increases fatty acid β-oxidation [23,24]. It is believed to be involved in improving insulin sensitivity and ameliorating hyperandrogenism associated with hyperinsulinemia in PCOS patients. Additionally, metformin treatment improves lipid profiles and endothelial function, decreases BMI, as well [25]. In vitro, it has also showed that metformin may affect androgen production by theca cells in ovarian [26]. Recently, a meta-analysis revealed that metformin led to a slightly reduction in fasting glucose level, systolic pressure and Waist-To-Hip Ratio (WHR). In our analysis, there are three studies, which all compared the treatment effects of acarbose and metformin in PCOS patients. We were unable to find out any differences between those two groups in fasting insulin, BMI, LH, FSH, and testosterone levels. In contrast, the pregnancy rate was significantly higher in acarbose group when analyzed the data from all three trials. In fact, when compared with control groups, both acarbose and metformin treatments were effective in improving ovulation rates, regular menstrual rates, or pregnancy rates. Only in clomiphene citrate-resistant PCOS patients, alleviated insulin resistance were observed in both acarbose and metformin group [15]. Discordant findings of studies evaluating the effects of these two agents on BMI have also been reported. BMI significantly decreased after treatment in both groups in Moini’s study [17], however, only one treatment group, acarbose or metformin group, has been found to reduce BMI in two remaining studies [11,15]. With regard to the gastrointestinal side effect, its frequency was as same as or slightly less than metformin. Therefore, acarbose may be an alternative to metformin for women with PCOS, especially for patients who are intolerant of metformin. The lowest clinical effect dose of acarbose is 150 mg/day and drug effectiveness will not be improved when greater than 300 mg/day is used [27]. In this analysis, most studies used a dose of 300 mg/day for acarbose treatment. Although the side effect of acarbose is dose-dependent and many patients had gastrointestinal complaints. It is still a mild drug and only a few patients dropped out from some studies. There are still some limitations in this meta-analysis. Only 7 RCTs were included in the analysis, and most of them were conducted in a small sample size, which may affect the reliability of the results. There are many differences among these studies, including study populations, methodologies for evaluating insulin resistance, doses of acarbose and control groups. Thus, the evidence of this meta-analysis may be not strong. Some studies did not describe the allocation concealment and whether a double-blind has been conducted. Therefore, large-scale, randomized, double-blinded, placebo-controlled studies of acarbose for the treatment of PCOS are needed for further investigations. In summary, treatment with acarbose, at the dose of 150-300 mg/d, has been found to improve various clinical manifestations of PCOS and it may be a safe and effective drug for these patients. Although very few subjects were dropped out in some studies due to its adverse effects, tolerability may still be an issue. It is too early to draw conclusions, as there are still lots of limitations in the currently available data.
Fundings
National Natural Science Foundation of China (81070651, 81170758, 81270946), National key; Research Seed Fund of Renji Hospital, School of Medicine, Shanghai Jiaotong University (RJZZ13- 027).
References
- Chen X, Yang D, Mo Y, Li L, Chen Y, Huang Y. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Biol. 2008; 139: 59-64.
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004; 89: 2745-2749.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999; 84: 4006-4011.
- Gurnell EM, Chatterjee VK. Dehydroepiandrosterone replacement therapy. Eur J Endocrinol. 2001; 145: 103-106.
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007; 370: 685-697.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014; 20: 748-758.
- Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013; 78: 782-785.
- Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997; 15: 111-122.
- Chang RJ. A practical approach to the diagnosis of polycystic ovary syndrome. Am J Obstet Gynecol. 2004; 191: 713-717.
- Kircher C, Smith KP. Acarbose for polycystic ovary syndrome. Ann Pharmacother. 2008; 42: 847-851.
- Hanjalic-Beck A, Gabriel B, Schaefer W, Zahradnik HP, Schories M, Tempfer C, et al. Metformin versus acarbose therapy in patients with polycystic ovary syndrome (PCOS): a prospective randomised double-blind study. Gynecol Endocrinol. 2010; 26: 690-697.
- Tugrul S, Kutlu T, Pekin O, Baglam E, Kiyak H, Oral O. Clinical, endocrine, and metabolic effects of acarbose, a alpha-glucosidase inhibitor, in overweight and nonoverweight patients with polycystic ovarian syndrome. Fertil Steril. 2008; 90: 1144-1148.
- Araujo Penna I, Canella PR, Vieira CS, Silva de SÁ MF, dos Reis RM, et al. Cardiovascular risk factors are reduced with a low dose of acarbose in obese patients with polycystic ovary syndrome. Fertil Steril. 2007; 88: 519-522.
- Penna IA, Canella PR, Reis RM, Silva de Sá MF, Ferriani RA. Acarbose in obese patients with polycystic ovarian syndrome: a double-blind, randomized, placebo-controlled study. Hum Reprod. 2005; 20: 2396-2401.
- Sönmez AS, Yasar L, Savan K, Koç S, Ozcan J, Toklar A, et al. Comparison of the effects of acarbose and metformin use on ovulation rates in clomiphene citrate-resistant polycystic ovary syndrome. Hum Reprod. 2005; 20: 175-179.
- Ciotta L, Calogero AE, Farina M, De Leo V, La Marca A, Cianci A. Clinical, endocrine and metabolic effects of acarbose, an alpha-glucosidase inhibitor, in PCOS patients with increased insulin response and normal glucose tolerance. Hum Reprod. 2001; 16: 2066-2072.
- Moini A, Eslami B. Comparision of menstrual pattern, clinical and hormonal effects of Acarbose with Metformin in polycystic ovarian syndrome. J Reproduktionsmed Endokrinol. 2010; 7: 369-370.
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989; 38: 1165-1174.
- Allahbadia GN, Agrawal R. Polycystic Ovary Syndrome. Kent, UK: Anshan, Ltd. 2007; 93–101.
- Kelly CJ, Stenton SR, Lashen H. Insulin-like growth factor binding protein-1 in PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2011; 17: 4-16.
- De Leo V, la Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003; 24: 633-667.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81: 19-25.
- Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009; 30: 1-50.
- Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010; 1205: 192-198.
- Diamanti-Kandarakis E, Alexandraki K, Protogerou A, Piperi C, Papamichael C, Aessopos A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur J Endocrinol. 2005; 152: 749-756.
- Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003; 79: 956-962.
- Rodier M, Richard JL, Monnier L, Mirouze J. Effect of long term acarbose (Bay g 5421) therapy on metabolic control of non insulin dependent (type II) diabetes mellitus. Diabete Metab. 1988; 14: 12-14.