Editorial
State of the art
According to recent scientific studies, we are currently suffering more unprecedented natural disasters caused by climate change (global warming), which is due to the emission of large amounts of greenhouse gases. How to reduce climate change is driving the political, social and technological agenda. In terms of greenhouse gas emissions, coal power generation makes up a large proportion of CO2 worldwide emissions. In order to minimize such emissions, a series of clean energy projects have been initiated for energy production with Carbon Capture and Storage (CCS). These projects can be classified into three categories: the Integrated Gasification Combined Cycle (IGCC) technology (pre-combustion), Oxyfuel, and post-combustion (requiring CO2 separation from the flue gases) [1]. Compared to the former two categories, post-combustion technology involving the CO2 separation stage is too expensive to justify economically, due to the vast amount of flue gas with diluted CO2. Comparatively, IGCC and Oxyfuel projects are more feasible. However, these two clean energy categories need oxygen as the feed gas. For instance, if pure oxygen instead of air is used in power plants, the major constituent of the waste gas produced during the combustion process would be CO2, which can be easily and economically captured. In the oxygen market, consumption has been dominated by conventional industries like metal manufacturing, chemicals, pharmaceuticals, petroleum, glass, cement, ceramics, pulp/paper manufacturing and others; the power generation is only sharing 4% [2]. However, in the near future, this market for energy will be massively expanded by the deployment of these clean energy projects. Current tonnage O2 production by a cryogenic process is very expensive and energy intensive. The addition of an extra cryogenic air separation unit in the coal gasification or Oxyfuel power plant is impractical because of its high capital investment and operational cost. Membrane technology is becoming more and more attractive for a possible energy-efficient gas separation method. Due to their lower selectivity, polymeric membranes have been ruled out for pure oxygen production purpose but they have applications in getting oxygen-enriched atmosphere. Oxygen and nitrogen have very much similar kinetic diameters (O2: 0.346 nm; N2: 0.364 nm), therefore it is difficult to perform the air separation using microporous molecular sieving membranes like zeolite/silica membranes. Fortunately, the oxygen selective membranes can be made from dense ceramic membranes. Dense Mixed Ionic and Electronic Conducting (MIEC) ceramic membranes can deliver 100% pure O2 under differential O2 partial pressure gradients without the requirement of external electric power, offering the potential to reduce the separation cost and improve the viability of these clean energy technologies. From the perspective of application, membranes must possess sufficiently high oxygen flux value and good structural stability to withstand practical conditions. In real applications, the oxygen permeation performance is dependent on several factors such as the material composition, the membrane thickness and operating conditions [3].
Among the reported ceramic materials with MIEC properties, perovskite and fluorite oxides have attracted most attention from researchers as they display high oxygen flux values, in particular the former membranes. The general formula of the perovskite is ABO3 in which the metal cations at A sites are larger than the B site cations (Figure 1a). The framework of perovskite ABO3 is very similar to ReO3, consisting of the corner-sharing BO6 octahedra with A cations located in twelve-coordinated interstices, as shown in (Figure 1a). Such an oxygen vacancy-disordered cubic perovskite structure is favourable for oxygen ion movement and electron conduction thus displaying the highest oxygen flux. In order to maintain the cubic perovskite structure with sufficient oxygen vacancy concentration, partial replacement (also called doping) of A or B cations in the ABO3 by other cations with different ionic radius, lower valence states and lower metal-oxygen bond energy is frequently performed. So far, a series of perovskite oxides with formula AyA'(1-y)BxB'(1-x)O(3-a) of have been reported to possess appreciably high oxygen permeation rates. In this formula, the A/A' belongs to the group of the alkaline-earth or rare-earth elements consisting of La, Sr, Ba, or Ca; and the B/B' is largely taken from the group of transitional metals consisting of Sc, Cr, Mn, Fe, Co, Ni, Cu, Ga, Zr, or Zn [4]. Actually, perovskite ABO3 has a very large flexibility to accommodate many other metal elements thus 90% of the metal elements in the periodic table have been attempted to be incorporated inside the perovskite structure to tune the properties to suit different applications. Among this series, typical examples are Ba0.5Sr0.5Co0.8Fe0.2O3-d (BSCF) and La0.6Sr0.4 Co0.2Fe0.8O3-d (LSCF) developed from the parent material of SrCoO3 [5,6]. BSCF is featured by its higher oxygen flux values but lower stability; on the contrary, LSCF is characterised by its moderate flux but higher stability. If the membrane is considered for other purposes more than the pure oxygen production from air, it may require higher stability to tolerate the practical atmosphere. As such, we have to consider the different susceptibility of perovskite oxides to react with other gases like H2O, CO2, H2 and CH4. If these gases are present in a sufficiently high concentration, they quickly poison the perovskite membrane surface and fail the function of oxygen transport. To optimize the membrane for oxygen separation, in addition to the material selection, engineering considerations should also be given, which will be discussed from (Figure 1b).
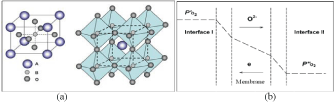
Figure 1: Schematic illustration of the structure of perovskite ABO3 (a: left column: one B-site cation at the centre of the cell and right column: one A-site cation
located in twelve coordinated interstices with corner-sharing (BO6) octahedral) and five major steps involved in oxygen permeation through the MIEC membrane (b).
As can be seen (Figure 1b), oxygen permeation through a MIEC membrane from the high to the low oxygen concentration sides involves five major steps in series: 1) Oxygen transfer from the gas stream to the membrane surface (interface-I at the high pressure side); 2) Surface exchange reactions between the molecular oxygen and oxygen vacancies at the membrane surface; 3) Oxygen ion bulk diffusion across the membrane; 4) The second surface reaction between lattice oxygen and electron at the membrane surface (low pressure side); and 5) Oxygen transfer from the membrane surface to the gas stream (low pressure side). Generally, the resistance from step (1) and (5) can be negligible compared to other steps, thus the overall oxygen flux rates can be described by following equation [7].
Jo2(flux)=driving force/resistance=Δ(Po2)/R1ex+R2diff+R3ex=Δ(Po2)/ Roverflow(tem)
From this equation, the driving force is from the oxygen partial pressure gradient and temperature and a high value of both will promote the oxygen flux. In particular, the higher operating temperature will greatly increase the oxygen ion diffusion rate and facilitate the surface exchange reactions. The resistances are largely from the two oxygen exchange reactions and bulk diffusion. Theoretical studies can be carried out to determine the resistance percentage from each step in the overall resistance, thus the controlling step for oxygen transport can be identified. For perovskite membranes in the thickness range from 100 microns to 1 mm, the oxygen transport is jointly controlled by the bulk diffusion and surface reactions. With this in mind, more strategies from an engineering perspective to increase the oxygen flux are to reduce the membrane thickness and to make the surface area more porous or deposit a catalyst layer on the surface to increase the surface reaction kinetics. Membrane geometry is also a factor that should be considered.
Currently, most of the related research work in MIEC membranes for fundamental studies is using disk-shaped membranes with very limited area for oxygen permeation. Relative to the tubular membranes, these disk-shaped membranes are easily prepared by the conventional static-pressing method. Due to the smaller membrane surface area per unit volume, these disk-shaped or large tubular membranes are unfavourable in practical applications. Alternatively, hollow fiber membrane exhibits many advantages as this geometry can provide the largest membrane area per unit volume and the natural separation of the two membrane surfaces thus simplifying the sealing issues usually encountered in the disk-shaped membrane design. The well-established immersion-induced phase inversion method, commonly employed to prepare polymeric hollow fiber membranes, has been successfully modified to prepare ceramic hollow fiber membranes. The resultant perovskite hollow fibre membranes have the inherent asymmetric structure, a thinner layer self-supported on porous substrate in the same membrane material, ideally for oxygen separation [8]. The only disadvantage is the low mechanical strength of these ceramic hollow fibers. Recently, one good strategy has been reported to bind several hollow fibre membranes together into one single body to increase the mechanical strength; and simultaneously the oxygen flux values are improved, as the binder is the porous perovskite materials that can be used as the catalyst to increase the surface reaction kinetics [9].
Future works
For the sole purpose of oxygen production from air separation, the perovskite membranes have been successfully developed with an oxygen flux of more than 10 ml cm-2 min-1 (the minimum target for commercial consideration) being reported. These perovskite membranes can be operated for more than 1000 hours at relatively mild air separating conditions. To realise their large scale applications, some challenging issues for membrane module scale up without defects and high temperature sealing still needs to be worked out. However, there is a long way to realize these ceramic membranes as membrane reactors for greener chemical synthesis. Such catalytic membrane reactor for the partial oxidation of methane to syngas has been referred as the revolutionary technology to combine the oxygen production and syngas generation in a single unit operation as it bring about significant benefits in both capital cost and energy savings [10]. The existing perovskite membranes can rarely tolerate the high temperature atmosphere containing H2O, CO2, H2 and CH4. The reactions between the perovskite oxides and the gases will destroy the perovskite structure. By contrast, the pure ion conducting fluorite oxides like Yttria-Stabilized Zirconia (YSZ), Sm-Doped Ceria (SDC) or Gd-Doped Ceria (GDC) possess inherently high chemical stability against these gases and can be used in the reacting conditions. Unfortunately, these robust pure ion conducting ceramics do not have the electronic conduction to satisfy the conditions to be used as MIEC membranes. Thus, these fluorite ceramics can only be used as dual phase membranes which can be prepared by combining one of these fluorites for ion conduction and another electronic conducting phase like one of the precious metals of Ag, Pd, Au, or Pt. Each of the conducting properties requires a continuous material pathway, which poses challenges to the preparation protocol regarding the mixture portions, mixing method, the particle size of the chosen membrane materials. The mismatch of these factors usually leads to the very low oxygen permeation fluxes, which is blocking their real applications. Fortunately, a new dual membrane design via the external short circuit has been put forward to minimise the usage of the electronic conducting phase and to maximise the oxygen flux values [11]. Such a new dual membrane design has been recently expanded from a fluorite disk-shaped membrane to a hollow fibre configuration. These robust membranes will undoubtedly open new windows for more advanced applications like as the high temperature membrane reactors for chemical synthesis from natural gas. Of course, to realise their real applications still requires significant research development and the joint efforts from the material scientists and chemical engineers.
References
- Kneer R, Toporov D, Forster M, Christ D, Broeckmann C, Pfaff E, et al. OXYCOAL-AC: towards an integrated coal-fired power plant process with ion transport membrane-based oxygen supply, Energy Environ Sci. 2010; 2: 198-207.
- ‘Clean Coal’ Technologies, Carbon Capture & Sequestration. Energy & environment. 2017.
- Sunarso J, Baumann S, Serra JM, Meulenberg WA, Liu S, Lin Y, et al. Mixed Ionic-Electronic Conducting (MIEC) ceramic-based membranes for oxygen separation. J Membr Sci. 2008; 320: 13-41.
- Zhang K, Sunarso J, Shao Z, Zhou W, Sun C, Wang S, et al. Research progress and materials selection guidelines on mixed conducting perovskitetype ceramic membranes for oxygen production. RSC Adv. 2011; 1: 1661- 1676.
- Shao Z, Yang W, Cong Y, Dong H, Tong J, Xiong G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3-d oxygen membrane. J Membr Sci. 2000; 172: 177-188.
- Lane J, Benson SJ, Waller D, Kilner JA. Oxygen transport in La0.6Sr0.4Co0.2Fe0.8O3-d, Solid State Ionics. 1999; 121: 201-208.
- Xu SJ, Thomson WJ. Oxygen permeation rates through ion-conducting perovskite membranes. Chem Eng Sci. 1999; 54: 3839-3850.
- Liu S, Gavalas GR. Oxygen selective ceramic hollow fiber membranes. J Membr Sci. 2005; 246: 103-108.
- An R, Song J, Li Y, Tan X, Sunarso J, Zhang C, et al. Bundling strategy to simultaneously improve the mechanical strength and oxygen permeation flux of the individual perovskite hollow fiber membranes. J Membr Sci. 2017; 527: 137-142.
- Repasky J, McCarthy D, Armstrong P, Carolan M. ITM Technology for Carbon capture on natural gas and hydrid power systems. Workshop on Technology Pathways Forward for Carbon Capture & Storage on Natural Gas Power Systems. Organized by U.S. Energy Association Supported by U.S. Dept of Energy Washington. DC. USA. April 22. 2014.
- Zhang K, Shao Z, Li C, Liu S. Novel CO2-tolerant ion-transporting ceramic membranes with an external short circuit for oxygen separation at intermediate temperatures. Energy Environ Sci. 2012; 5: 5257-5264.
